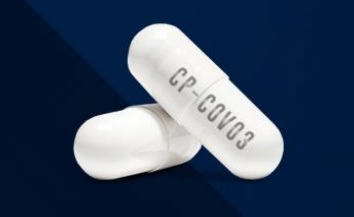
On the 13th, Hyundai Bio announced that in the Phase 2 clinical trial of 'CP-COV03,' a broad-spectrum antiviral agent under development, the maximum blood drug concentration (Cmax) of all blood collection participants exceeded the antiviral efficacy concentration (IC50, 100ng/㎖) required to inhibit the COVID-19 virus.
According to the top-line and pharmacokinetic (PK) data disclosed by Hyundai Bio, even the participant with the lowest blood drug concentration of CP-COV03 among those who had blood drawn 3 hours after administration exceeded the IC50 at 129.39ng/㎖. The average blood drug exposure (AUC) was higher in the high-dose (450mg) group than in the low-dose (300mg) group.
The company explained, "This has solved the bioavailability challenge that has blocked the drug repositioning of niclosamide, the main ingredient of CP-COV03, for over 60 years, for the first time in the world." Niclosamide, launched in 1959, has shown antiviral efficacy in various cell experiments, but its extremely low bioavailability has been a major obstacle to drug repositioning.
Jin Geun-woo, head of research at Hyundai Bio, said, "CP-COV03 shortened the overall improvement time for 12 symptoms by 4 days in the Phase 2 clinical trial for COVID-19," adding, "Our goal is to make CP-COV03 a broad-spectrum antiviral agent."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














