Ministry of Health and Welfare's 'Biohealth New Industry Regulatory Innovation Plan'
Comprehensive Regulatory Reforms in Medical Devices, Biopharmaceuticals, and Digital Health
 Minister of Health and Welfare Cho Kyu-hong is conducting a briefing on the 'Biohealth New Industry Creation Strategy.'
Minister of Health and Welfare Cho Kyu-hong is conducting a briefing on the 'Biohealth New Industry Creation Strategy.' [Photo by Ministry of Health and Welfare]
The government, which announced a new market creation strategy for biohealth, is now accelerating the development of related industries by presenting regulatory innovation measures. Through this, it aims to accelerate the market entry of innovative medical devices and establish a new model that concurrently conducts product approval and price negotiations for rare disease treatments. Along with the enactment of the so-called 'Digital Healthcare Act,' it is enhancing competitiveness across the entire biohealth sector by resolving regulations related to advanced biopharmaceuticals.
On the 2nd, the Ministry of Health and Welfare announced the 'Biohealth New Industry Regulatory Innovation Plan' containing these details at the 3rd Regulatory Innovation Strategy Meeting chaired by Prime Minister Han Duck-soo. This regulatory innovation plan was prepared at the government-wide level as part of the biohealth new market creation strategy announced on the 28th of last month, aiming to establish a foundation for becoming a global hub in digital and biohealth.
The Ministry of Health and Welfare will promote regulatory innovation in seven core areas that can protect citizens' lives and induce private innovation: ▲innovative medical devices ▲innovative and essential pharmaceuticals ▲digital healthcare ▲advanced regenerative medicine and advanced biopharmaceuticals ▲genetic testing ▲brain-machine interface ▲infrastructure.
First, to establish an advanced market pre-entry system for innovative medical devices, the scope of application will be further expanded beyond the existing new medical technology evaluation exemption system and integrated review and evaluation system. In particular, to enable the recently approved first domestic 'Digital Therapeutics (DTx)' to be utilized and spread in the domestic market, a plan for health insurance coverage will be established. In the innovative and essential pharmaceuticals sector, a pilot project will be conducted to simultaneously proceed with product approval, reimbursement evaluation, and price negotiation for cancer and rare disease treatments, and a public-private consultative body will be formed to prepare guidelines for remote clinical trials and appropriate compensation for innovative new drugs.
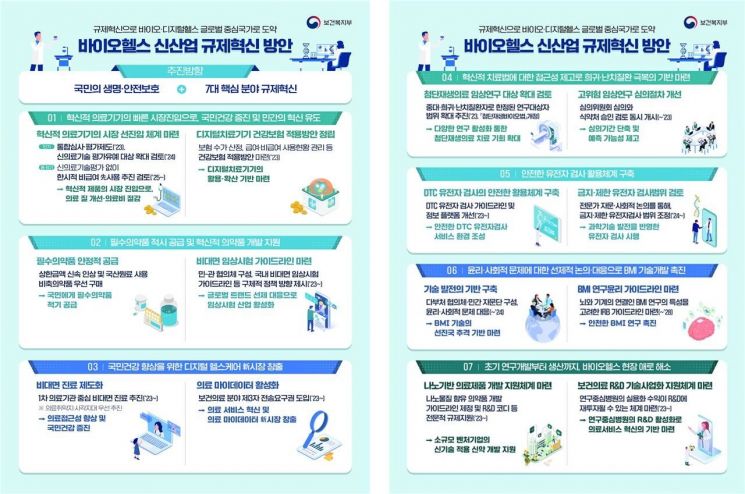 Infographic on Regulatory Innovation Measures for New Biohealth Industries. [Photo by Ministry of Health and Welfare]
Infographic on Regulatory Innovation Measures for New Biohealth Industries. [Photo by Ministry of Health and Welfare]
In the digital healthcare field, legislation will be pursued to allow medical institutions to directly transmit medical data to third parties who meet safety management standards, and guidelines for Institutional Review Boards (IRB) specialized in biohealth data will be prepared to establish a safe healthcare data utilization system. Regarding advanced regenerative medicine and advanced biopharmaceuticals, in the short term, the review process for high-risk clinical research will be improved to shorten the duration, while in the mid-to-long term, the introduction of regenerative medical procedures and the expansion of clinical research target diseases will be considered.
With the implementation of the consumer-directed testing (DTC) genetic testing certification system in July last year, domestic DTC genetic testing is expected to be activated, and related regulatory improvements will also be pursued. The DTC genetic testing guidelines will be revised to be practical for consumers and testing institutions, and the testing information platform functions will be strengthened to enable counseling and education. In the brain-machine interface (BMI) field, which controls devices through brain signals, specialized guidelines will be prepared, and a multi-ministerial consultative body and private advisory group will be formed to promote full-cycle technology development. Lastly, in terms of infrastructure, efforts will be made to establish a support system for the development of pharmaceuticals applying new technologies, install medical technology industry-academic cooperation groups, and strengthen support for startup companies by easing restrictions on production items and leasing for resident companies in advanced medical complex districts.
Minister of Health and Welfare Cho Kyu-hong said, "Biohealth is a future growth industry in an era of low growth and plays a pivotal role in national health and security," adding, "The Ministry of Health and Welfare will continue to promote regulatory innovation in the biohealth sector to create new biohealth markets and provide better healthcare services to the public."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














