[Asia Economy Reporter Lee Gwan-ju] The Ministry of Food and Drug Safety announced that it will attend the 'Global Harmonization Working Party (GHWP)' held in Riyadh, Saudi Arabia from the 13th to the 16th to strengthen the international cooperation network in the medical device sector and explore ways to support the export of domestic products.
GHWP is a cooperative organization launched in 1996 for the international harmonization of medical device regulations, currently involving 32 member countries worldwide, including the United States and Singapore. Currently, Korea's Ministry of Food and Drug Safety serves as the vice-chair of the Technical Committee and the chair of the Working Group.
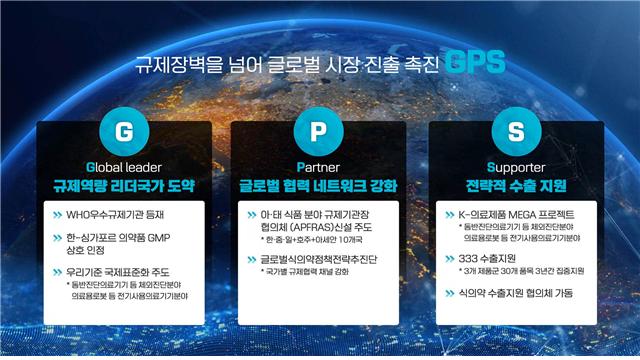
This GHWP attendance is part of the 'GPS strategy' to enable excellent domestic products to expand globally. At this meeting, the Ministry plans to challenge the reappointment as chair of the Working Group on General Medical Device Approval to promote the international common guidelineization of the Ministry’s guidelines. If domestic guidelines become international common guidelines, products approved domestically will also comply with international standards, facilitating smoother exports.
Additionally, the Ministry will introduce regulatory innovation cases in the digital health sector and guidelines for digital therapeutics and artificial intelligence. In particular, it plans to propose adopting the 'Guideline for Approval Review of Artificial Intelligence-Based In Vitro Diagnostic Medical Devices (Software),' established last December, as an international common guideline.
A Ministry of Food and Drug Safety official said, "We will continue to actively promote the GPS strategy based on regulatory science expertise to lead international regulatory harmonization in the medical device field and support the overseas market entry of excellent domestic medical devices."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.