IBS Confirms Mechanism of Amyloid Beta Production
[Asia Economy Reporter Kim Bong-su] The Institute for Basic Science (IBS) announced on the 10th that it has elucidated the formation mechanism of amyloid-beta, known as the main cause of cancer and Alzheimer's dementia.
The research team led by Jinwoo Cheon, head of the Nanomedicine Research Division (professor at Yonsei University Department of Chemistry and the Institute for Advanced Study), and research fellow Minseok Kwak (professor at Yonsei University and the Institute for Advanced Study) collaborated with Professor Youngwook Jeon of the University of California, San Francisco (UCSF), a visiting professor at the Nanomedicine Research Division.
The team used nanotechnology and cellular engineering techniques to clarify the activation process of the Notch receptor signaling, which plays a crucial role in tissue development, and the formation mechanism of amyloid-beta (Aβ), known as the main cause of Alzheimer's disease. Notch signaling has been known as an important intercellular interaction that regulates cell division and neurogenesis. Abnormal Notch signaling is a direct cause of various diseases, especially cancer. Additionally, amyloid-beta formed from amyloid precursor protein (APP) accumulates in tissues, causing neural damage and is known to be involved in the onset of Alzheimer's disease.
Interestingly, both Notch activation and amyloid-beta formation occur through a sequential proteolysis process of the Notch receptor and amyloid precursor protein by two different types of enzymes located in the cell membrane. Therefore, identifying the factors involved in this cleavage process and understanding the control mechanisms are essential for understanding vital biological phenomena such as stem cells and tissue development, as well as for the prevention and treatment of diseases like cancer and Alzheimer's. However, relatively little is known about the molecular mechanisms that precisely regulate the spatiotemporal order of the sequential cleavage process and the substrate specificity of the enzymes.
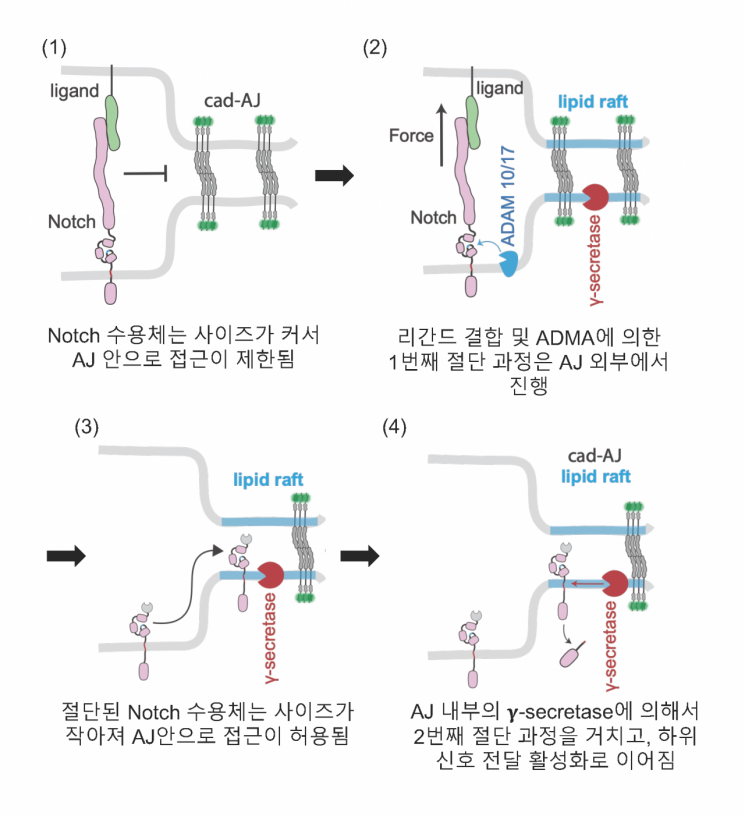
The research team discovered that adherens junctions (AJs), structures that control cell-to-cell adhesion, act as spatial switches determining the order of the sequential cleavage process and are essential for normal Notch signal regulation. Specifically, they found that the interaction between the Notch receptor and its ligand and the first cleavage of the receptor occur outside the adherens junction structure, while the second cleavage occurs within the adherens junction.
Before activation, the size of the Notch receptor is larger than the width of the adherens junction, limiting its access inside the junction. The Notch receptor, upon binding to the ligand and initiating activation, is cleaved by ADAM enzymes outside the adherens junction, reducing its size and allowing it to access inside the junction. Subsequently, the Notch receptor entering the adherens junction undergoes the second cleavage by γ-secretase enzymes, which are highly concentrated inside, leading to signal activation.
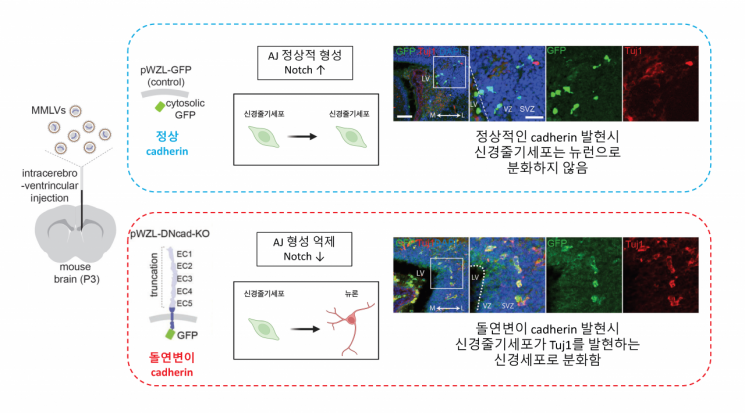 Relationship between differentiation of neural stem cells into neurons and formation of adhesive junctions. Photo by IBS
Relationship between differentiation of neural stem cells into neurons and formation of adhesive junctions. Photo by IBS
The researchers used mechanogenetics nanotechnology, which can deliver mechanical and spatiotemporal stimuli to specific receptors, to confirm that adherens junctions recruit γ-secretase enzymes at high concentrations and block access of Notch receptors that have not undergone the first cleavage. In other words, they confirmed that adherens junctions function to prevent abnormal receptor-enzyme interactions and Notch activation.
To verify this, they used CRISPR-Cas gene editing technology to remove the expression of cadherin proteins, which play a key role in forming adherens junctions and maintaining intercellular adhesion, and confirmed that Notch signaling was not activated. Furthermore, when cadherin expression was suppressed in neural stem cells of developing mouse brains, the stem cells abnormally differentiated into neurons at an accelerated rate. This demonstrated that the Notch signal regulation process by adherens junctions is involved in neural development.
Moreover, when adherens junction formation was inhibited in cells expressing amyloid precursor protein, the amount of amyloid-beta formed decreased. This showed that controlling the protein cleavage process can suppress the formation of amyloid-beta, known as a major cause of Alzheimer's disease.
Professor Youngwook Jeon stated, "We have presented for the first time a new molecular and cellular mechanism of the sequential cleavage process of proteins required for Notch signal activation and amyloid-beta formation," adding, "This research is the result of successfully applying mechanogenetics nanotechnology developed through joint research by the IBS Nanomedicine Research Division, Yonsei University Institute for Advanced Study, and UCSF to cell signaling research."
Research fellow Minseok Kwak said, "This study is expected to greatly contribute to future cancer-related research caused by abnormal cell signaling and Alzheimer's disease treatment research through inhibition of amyloid-beta formation," and explained, "We plan to expand the research by developing brain organoids that accurately mimic neural development processes by regulating Notch signaling."
The research results were published online on December 2 last year in the international journal Nature Cell Biology (IF 28.82).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














