Moderna's 'mRNA-4157' Combined with Keytruda in Clinical Trial
44% Reduction in Recurrence and Death Risk Compared to Keytruda Alone
Global Big Pharma Competes to Secure Technology
Domestic Companies Aston Science and Genexine Also Developing
[Asia Economy Reporter Lee Chun-hee] The era of treating cancer through vaccines is approaching. Moderna, a messenger ribonucleic acid (mRNA) vaccine specialist company, is developing cancer vaccines following COVID-19, and interest is growing as positive clinical results emerge. While global big pharma companies are rushing to secure technology, such as Moderna partnering with MSD (Merck & Co., USA), domestic development attempts are also ongoing.
On the 13th (local time), Moderna announced that its personalized cancer vaccine (PCV) 'mRNA-4157/V940' showed a "statistically significant and clinically meaningful reduction in the risk of disease recurrence or death" in combination therapy with MSD's immuno-oncology drug 'Keytruda' in the Phase 2 clinical trial (KEYNOTE-942).
In this trial, mRNA-4157 demonstrated efficacy by reducing the risk of recurrence and death (RFS) by 44% compared to the control group receiving Keytruda alone. Earlier, in October, MSD exercised its option for co-development and commercialization of mRNA-4157, paying Moderna $250 million (approximately 324.5 billion KRW), reflecting high expectations that have now been confirmed by actual results.
Regarding side effects, serious treatment-related adverse events occurred in 14.4% of the treatment group and 10% of the control group, showing no significant difference. The company explained that this was consistent with side effects reported in Phase 1 trials. Moderna plans to disclose more detailed data soon through academic conferences and aims to start Phase 3 clinical trials next year.
Following the release of these results, Moderna's stock closed at $197.54, soaring 19.63% compared to the previous day's closing price.
Cancer treatment vaccines work by inducing or amplifying the immune response of cancer patients through vaccination to enhance therapeutic effects. mRNA-4157 induces T-cell responses based on tumor mutations. It is a 'personalized vaccine' that creates vaccines tailored to the mutation characteristics of each patient's tumor sample.
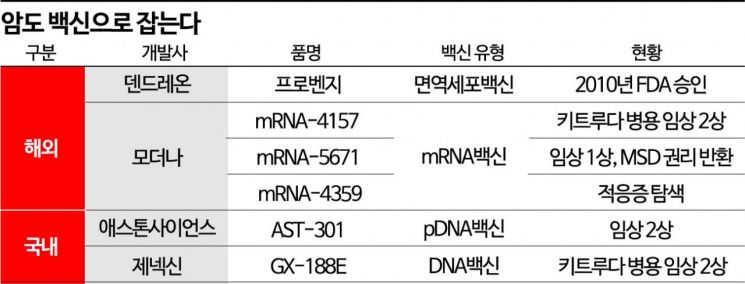
Stefan Bancel, CEO of Moderna, said, "Today's results are very encouraging in the field of cancer treatment," adding, "For the first time ever, mRNA has demonstrated the potential to impact melanoma in a randomized clinical trial." He also stated, "We will begin additional studies on melanoma and other forms of cancer with the goal of providing personalized cancer therapies." In fact, Moderna is also developing the KRAS mutation cancer vaccine 'mRNA-5671' and the PD-L1 targeted cancer vaccine 'mRNA-4359.' mRNA-5671 is currently in Phase 1 clinical trials, and mRNA-4359 is in the indication exploration stage.
So far, the only approved cancer vaccine is Dendreon's 'Provenge,' which received FDA approval in 2010 for prostate cancer. However, commercialization faced difficulties, pushing Dendreon to the brink of bankruptcy at one point, and ownership changed several times.
However, with the COVID-19 pandemic, various vaccine platforms such as mRNA and DNA have emerged, renewing the potential for cancer vaccine development. Market research firm Allied Market Research predicts that the global cancer vaccine market will grow from $3.345 billion (approximately 4.3418 trillion KRW) in 2020 to $7.303 billion (approximately 9.4798 trillion KRW) by 2027, with an average annual growth rate of 14.6%.
Big pharma companies are also paying attention, intensifying competition to secure technology. BioNTech of Germany, which developed the mRNA COVID-19 vaccine 'Comirnaty' with Pfizer, is conducting a Phase 2 clinical trial combining the mRNA-based cancer vaccine 'RO7187457' with Genentech's immuno-oncology drug 'Tecentriq' (active ingredient atezolizumab) together with Genentech (a Roche subsidiary). Additionally, BioNTech is conducting combination clinical trials of 'BNT111' and 'BNT116' with Regeneron's immuno-oncology drug 'Libtayo' in collaboration with Sanofi.
In South Korea, movements to develop cancer vaccines are also emerging. Aston Science has entered clinical trials with various plasmid DNA (pDNA)-based cancer vaccines such as 'AST-301,' 'AST-302,' and 'AST-201.' AST-301 is conducting Phase 2 combination therapy trials with Keytruda and others targeting HER2 low-expression breast cancer patients in the US, Australia, and Taiwan. AST-302, which targets multiple antigens for breast cancer patients, recently secured antigen-specific immunogenicity in 80% of patients in Phase 1 trials.
Genexine recently disclosed results from a Phase 1b/2 clinical trial combining its DNA vaccine 'GX-188E' with Keytruda. The median overall survival (mOS) increased nearly twofold to 17 months compared to 9 months with Keytruda monotherapy, with an objective response rate (ORR) of 31.7% and a complete remission rate of 10%. On the 8th, the company also announced that it is discussing procedures with the Ministry of Food and Drug Safety for designation as a fast-track target and conditional approval application.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.