Professor Sangyoung Lee's Yonsei University Team, Can They Replace Lithium-Ion Batteries?
[Asia Economy Reporter Kim Bong-su] A 'water-based zinc-ion battery' that does not explode and has a lifespan three times longer than conventional lithium-ion batteries has been developed.
Professor Lee Sang-young of Yonsei University, through an international joint research collaboration with Professor Kwak Sang-gyu of Korea University and Professor Stefano Passerini of Karlsruhe Institute of Technology in Germany, developed a water-based zinc-ion battery that is explosion-proof, inexpensive, and has a lifespan characteristic more than three times better than existing lithium-ion batteries. This research is expected to contribute to improving the lifespan characteristics and safety of large-capacity energy storage systems (ESS), which have recently gained attention due to communication outages caused by the Kakao server fire. Notably, it is significant in that it academically presents a new electrolyte design principle for the commercialization of zinc-ion batteries, which are receiving worldwide attention.
Currently, lithium-ion batteries dominate the market but face issues such as global supply instability of materials, high manufacturing costs, and safety problems like explosions and fires, necessitating next-generation battery systems that fundamentally resolve these issues. Zinc-ion batteries are based on the electrochemical storage mechanism of zinc ions. In particular, they use zinc metal?one of the abundant and inexpensive metal resources on Earth?as the anode and water as the electrolyte, which poses no explosion risk, making them safer and more cost-competitive compared to lithium-ion batteries.
However, zinc metal anodes face several challenges for commercial use. Zinc metal anodes corrode in water-based electrolytes, and hydrogen gas is generated due to the reductive decomposition of water, increasing resistance inside the battery. During charge and discharge cycles, zinc metal grows into sharp dendritic crystals, causing rapid efficiency degradation and internal short circuits. To address these issues, recent approaches have proposed surface modification or zinc metal composite methods to limit contact between water and zinc metal, but these are limited by complex processes and high costs. Various new electrolyte materials that can reduce water reactivity have also been studied, but they have encountered battery lifespan limitations and have not reached commercialization levels.
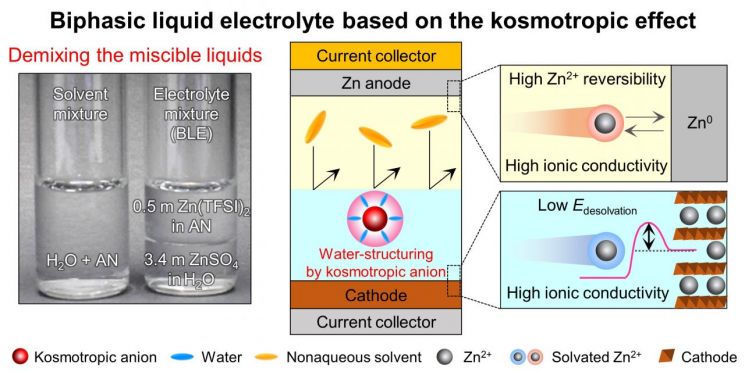
As a differentiated attempt to overcome these existing limitations, Professor Lee Sang-young’s research team at Yonsei University developed a customized biphasic electrolyte for zinc-ion batteries. A biphasic electrolyte refers to two different types of electrolytes coexisting without mixing, like water and oil. By controlling molecular interactions within the electrolyte, they realized a biphasic electrolyte optimized for the operating environments of both the cathode and anode.
The team’s biphasic electrolyte effectively suppressed corrosion and dendrite growth of the zinc metal anode, achieving a high charge-discharge efficiency of 99.6%, while simultaneously enhancing the reaction rate at the cathode. It also enabled rapid ion transport within the electrolyte. Through this, they realized a battery that does not explode, is cost-competitive, and has a lifespan characteristic about three times superior to conventional lithium-ion batteries, demonstrating the practical commercialization potential of zinc-ion batteries.
Professor Lee said, “This research not only dramatically improved the energy density and lifespan of zinc-ion batteries using zinc materials and water-based electrolytes free from global raw material supply chain issues, but also presented a new direction for electrolyte design that can maximize battery performance. We expect it to be applied in various fields such as large-capacity energy storage systems and wearable devices, where high safety is required.”
The research results were published on the 1st in the international academic journal Energy & Environmental Science (IF: 39.714).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














