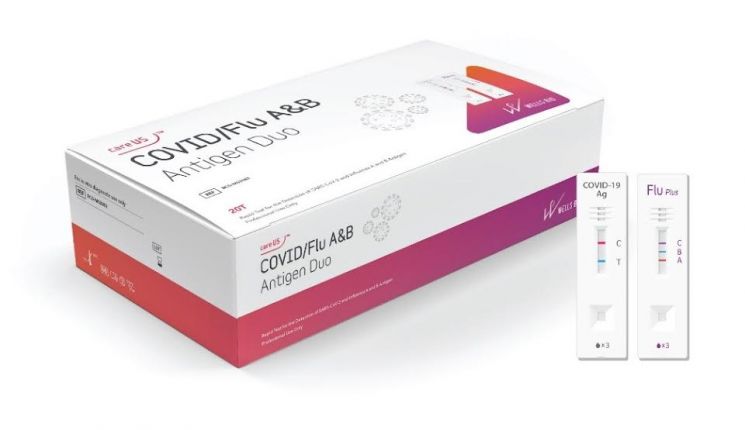
[Asia Economy Reporter Lee Chun-hee] Access Bio announced on the 19th that its subsidiary Wells Bio has obtained domestic approval for the diagnostic kit ‘careUS COVID/Flu A&B Antigen Duo,’ which can simultaneously detect COVID-19 virus and influenza A and B viruses.
This kit is an in vitro diagnostic medical device that helps diagnose infections by testing for SARS-CoV-2 antigen and influenza virus types A and B antigens from nasopharyngeal swab specimens of suspected patients with respiratory infection symptoms using immunochromatography. It was designed to prepare for the "twindemic" (simultaneous outbreak of COVID-19 and flu). It is a professional medical product composed of the existing rapid antigen diagnostic kit for COVID-19 ‘careUS COVID-19 antigen’ and the rapid antigen diagnostic kit for influenza A and B ‘careUS™ Flu A&B Plus.’ It can be used when comprehensive diagnosis for COVID-19 and flu is needed under the judgment of medical professionals.
Additionally, this product allows test results to be confirmed within 15 minutes, enabling not only rapid reading but also swift patient response. In particular, the COVID-19 rapid antigen diagnostic kit among these products has completed verification against major Omicron variants (BA.2, 3, 4, 5, 2.75), securing performance reliability.
Last month, the Korea Disease Control and Prevention Agency issued a nationwide flu outbreak advisory for the first time since 2019, before the COVID-19 pandemic, raising concerns about the twindemic. In response, Wells Bio plans to officially launch the simultaneous diagnostic kit within the fourth quarter of this year and actively supply it to domestic medical institutions. They also plan to enter overseas diagnostic markets such as Europe through the CE certification currently being prepared.
A Wells Bio official stated, “With the prolonged COVID-19 pandemic and the issuance of a flu outbreak advisory, the sense of crisis at the national quarantine level is rising again,” adding, “As a specialized in vitro diagnostic medical device company, we will take great responsibility and continuously supply numerous high-performance diagnostic kits to positively influence the domestic quarantine system.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.