MFDS Verifies Quality of SK Bioscience's 'Skycovione Multi'
[Asia Economy Reporter Jo In-kyung] On the 26th, the Ministry of Food and Drug Safety granted national batch release approval for 610,000 doses of 'Skycovione Multi,' the first domestically developed COVID-19 vaccine by SK Bioscience.
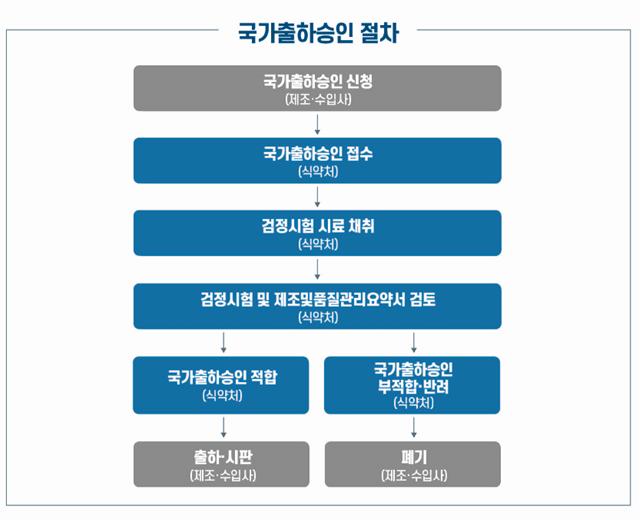
National batch release approval is a system where the state evaluates the results of inspection tests and the manufacturer's production and testing outcomes to verify the quality of pharmaceuticals containing substances derived from biological organisms, such as vaccines, before they are distributed in the market. Once national batch release approval is obtained, manufacturers can ship and sell their products.
Skycovione Multi is a vaccine manufactured using a recombinant gene technology method to produce the antigen of the COVID-19 virus. The Ministry of Food and Drug Safety decided to approve this vaccine at the end of June, and SK Bioscience applied for national batch release approval on the 12th.
The Ministry of Food and Drug Safety stated, "We conducted the national batch release approval based on scientific evidence with safety as the top priority," and added, "We will continue to verify COVID-19 vaccines quickly and thoroughly to ensure that vaccines with secured quality can be supplied stably."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















