Targeting the Market from Low to High Concentrations
Humira, a Mega Blockbuster with 27 Trillion KRW Sales Last Year
Intense Biosimilar Market Competition Starting July Next Year
[Asia Economy Reporter Chunhee Lee] Samsung Bioepis announced on the 18th that it had obtained approval from the U.S. Food and Drug Administration (FDA) on the 15th (local time) for the high-concentration formulation (100 mg/mL) of Hadlima, a biosimilar of Humira (active ingredient adalimumab).
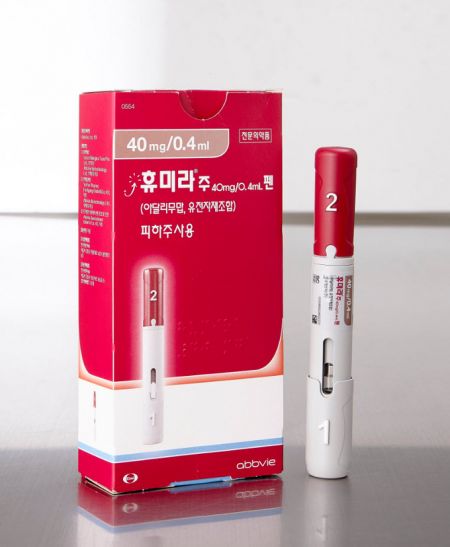
Humira, developed by AbbVie, is a treatment for rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, and plaque psoriasis. It has consistently ranked among the top global sales blockbuster drugs. It held the world's top sales position for nine consecutive years until 2020. Last year, its sales reached $20.694 billion (approximately 27 trillion KRW), but it lost the top spot to Pfizer's COVID-19 vaccine Comirnaty, which recorded $36.8 billion (approximately 48 trillion KRW) in sales. However, there is a strong possibility that it will regain the number one position next year.
Hadlima previously received FDA approval for its low-concentration (50 mg/mL) formulation in July 2019. Since its launch in Europe in 2018 (under the product name Imraldi) and in Korea last year (under the product name Adaloce), more than 5 million doses have been prescribed. Samsung Bioepis developed Hadlima in pre-filled syringe (PFS) and auto-injector forms to provide convenience for patients who self-inject.
Additionally, to meet on-site demand, a high-concentration formulation was developed. High-concentration products are known to be preferred by patients because they require a smaller volume of drug administration compared to existing products. AbbVie is also targeting the market by developing a high-concentration formulation of Humira.
The approval of the citrate-free high-concentration formulation of Hadlima was based on clinical trials conducted in Germany from August 2020 to May last year involving 188 healthy adult males. The study compared pharmacokinetics, safety, tolerability, and immunogenicity between the low-concentration (40 mg/0.8 mL) and high-concentration (40 mg/0.4 mL) products, demonstrating biological equivalence in pharmacokinetic properties and safety. Hadlima is scheduled to be launched in the U.S. market by Organon after July 1 next year.
Jung Byung-in, Head of Samsung Bioepis RA (Regulatory Affairs) Team and Executive Director, said, “With the FDA approval for the high-concentration formulation, we now have both low- and high-concentration Hadlima products, expanding treatment options for patients. We will continue to provide reasonable treatment opportunities using high-quality pharmaceuticals to patients worldwide by leveraging Samsung Bioepis’ expertise in research and development as well as production and supply chain management capabilities.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














