From Consultation on New Medical Technologies and Health Insurance Coverage
to Specialized Support Across Professional Fields
[The Asia Business Daily Yeongnam News Bureau, Reporter Yeo Jonggu] The Korean Medicine Innovative Technology Development Project Team at the National Institute for Korean Medicine Development will expand the 'PtoE Research Support' program to the entire Korean medicine community starting in August.
According to the National Institute for Korean Medicine Development on August 12, the 'PtoE (Practice to Evidence) Research Support Program' will now include not only clinical Korean medicine doctors and researchers, but also companies in the field.
The PtoE Research Support Program was initially launched to provide consultation on research methodology and institutionalization to strengthen the evidence base for research outcomes, targeting researchers involved in the Korean Medicine Innovative Technology Development Project.
Subsequently, the scope was broadened to the entire Korean medicine community, enabling research to be designed and prepared with professional support from the early stages.
The main consultation areas available for application include research methodology (such as clinical research design and analysis, economic evaluation, clinical trial protocol approval, development of standard clinical practice guidelines, big data analysis, and more) as well as strengthening and institutionalizing coverage (such as registration of new medical technologies, inclusion in health insurance benefits, obtaining product approvals, and planning public health projects).
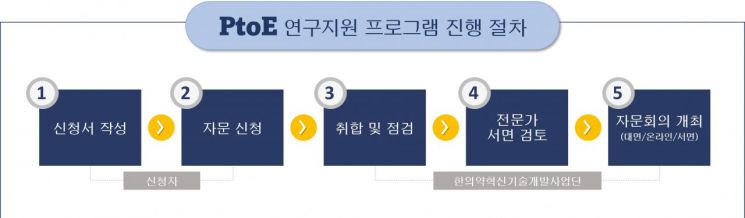
Those seeking consultation can download the application form from the 'National Korean Clinical Information Portal,' complete it, and submit it to the Korean Medicine Innovative Technology Development Project Team.
Park Minjeong, head of the Korean Medicine Innovative Technology Development Project Team, stated, "Although various studies have been conducted on clinically effective Korean medicine treatment technologies, there have been cases where the results did not meet expectations due to a lack of understanding of the health insurance or new medical technology systems. We will actively support the creation of diverse evidence to improve the quality of Korean medical services and strengthen coverage."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














