[Asia Economy Reporter Kim Young-won] The government has decided not to receive 16.65 million doses of Janssen and COVAX Facility (international vaccine supply project) COVID-19 vaccines that were scheduled to be introduced domestically this year.
On the 7th, the Central Disease Control Headquarters announced that in order to enhance the utilization of vaccines secured domestically, the inter-ministerial task force including the Korea Disease Control and Prevention Agency and the Ministry of Health and Welfare discussed and agreed to reduce the supply volume of Janssen and COVAX vaccines.
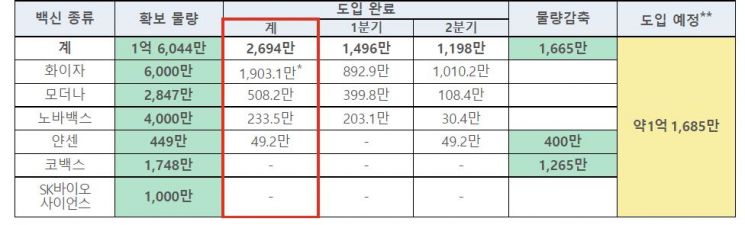
Out of the 160 million doses secured this year, 26.94 million doses have already been introduced. Including the carryover from last year, about 18 million doses of vaccines remain in the country.
Accordingly, the government has contracted to reduce the Janssen vaccine volume by 4 million doses. Since 490,000 doses out of the secured 4.49 million doses have already been introduced domestically, no additional Janssen supply is expected in the country.
Among the 20 million doses purchased through the COVAX Facility, it was also decided not to introduce an additional 12.65 million doses, and a refund procedure will be carried out. 2.52 million doses were introduced earlier, and 4.83 million doses of AstraZeneca (AZ) were already allocated in August last year.
The government explained that the Pfizer vaccine supply schedule was adjusted so that it would not be supplied in the third quarter when additional vaccinations are not conducted. It is also known that the Moderna vaccine supply schedule is under negotiation for adjustment. Additionally, 800,000 doses of Pfizer and 40,000 doses of Moderna will be supported to Mexico and Guyana.
The supply deadline for the Novavax vaccine has been adjusted until the end of next year. The first domestic vaccine, SK Bioscience's 'Skycovione,' is contracted until June 2024, and the supply timing will be determined based on domestic vaccination demand.
Furthermore, the government stated that if improved vaccines targeting variant viruses are released, they are in continuous consultation with pharmaceutical companies from the development stage to enable rapid introduction according to domestic demand.
A representative from the Central Disease Control Headquarters said, "We will continue to seek ways to maximize the use of vaccines both domestically and internationally, including additional consultations with pharmaceutical companies."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














