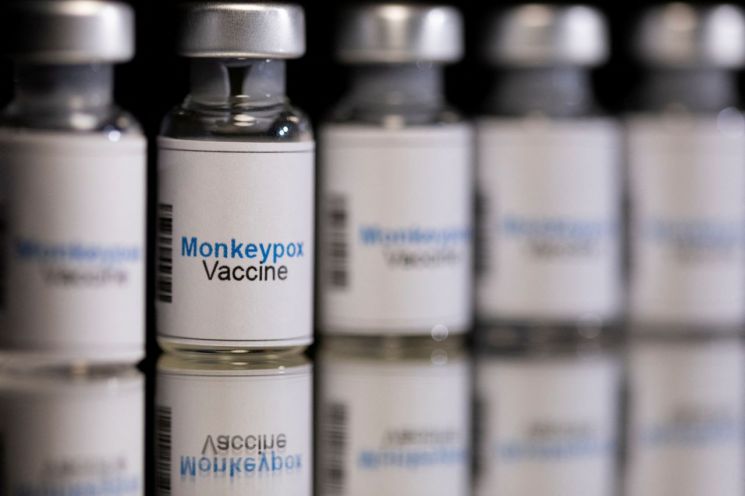
Contract Underway to Supply 5,000 Doses of 3rd Generation Smallpox Vaccine 'Jynneos'
[Asia Economy Reporter Kim Young-won] The antiviral drug used to treat monkeypox will be introduced in South Korea this week. The third-generation smallpox vaccine is also expected to be supplied domestically soon.
On the 5th, Lim Sook-young, head of the Central Disease Control Headquarters' situation management team, stated, "The Ministry of Food and Drug Safety approved the emergency import of the third-generation smallpox vaccine Jynneos on the 1st, and a supply contract is currently underway. Additionally, about 500 doses of the monkeypox treatment Tecovirimat will be imported and supplied to hospitals in cities and provinces."
Jynneos, a third-generation smallpox vaccine developed by Danish company Bavarian Nordic, was approved in the United States in 2019 for monkeypox prevention. While the second-generation vaccine has complicated administration methods and a relatively higher chance of adverse reactions, the third-generation vaccine can be administered via a standard subcutaneous injection, which is an advantage.
Tecovirimat is the only product approved overseas as a monkeypox treatment and can be used for adults and pediatric patients weighing 13 kg or more. After 504 doses are imported on the 9th, they will be supplied to designated hospitals in 17 cities and provinces nationwide for use.
However, health authorities report that many monkeypox patients exhibit mild symptoms and often improve without using the treatment. Lim said, "Even in the case of the first patient, we have been informed that the condition improved significantly without the use of a dedicated monkeypox treatment."
When asked if the amount of Tecovirimat being imported is sufficient, Lim responded, "The current quantity being imported is enough for initial response, and considering future developments, additional imports will be made if necessary."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














