[DTx Era ④] Dementia
No Clear Cause Identified, No Cure Available
Biogen's FDA-Approved Aduhelm Became an Issue but Failed Commercialization
As Life Expectancy Increases, Dementia Patients Also Rise
1.08 Million in 2025 · 3.02 Million in 2050
Treatment and Management Costs Expected to Reach 103 Trillion KRW
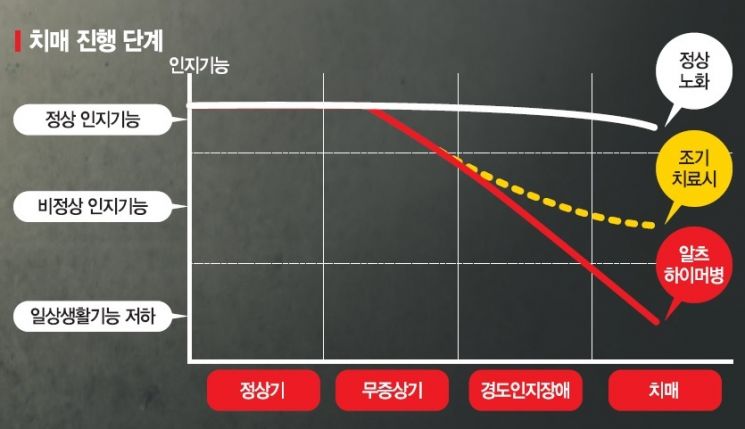
Proper Treatment During Mild Cognitive Impairment Can Prevent Rapid Disease Progression
[Asia Economy Reporter Chunhee Lee] ‘Dementia’ remains an unknown disease. Since the exact cause of the condition has not been identified, no proper treatment has been developed. Last year, Biogen’s antibody treatment ‘Aduhelm’ (generic name Aducanumab) gained sudden fame as a star treatment after receiving approval from the U.S. Food and Drug Administration (FDA), but it soon failed in commercialization, sparking controversy.
On the other hand, the outlook that the number of dementia patients will continue to increase is overwhelming. Given the high correlation between human aging and dementia, if proper treatments are not developed, the number of dementia patients will inevitably rise as human life expectancy increases.
According to the Central Dementia Center, the number of dementia patients in South Korea is expected to exceed 1.08 million in 2025, surpassing one million for the first time, and reach approximately 3.02 million by 2050, which is three times more. The cost of dementia treatment and management is also expected to increase exponentially from 17 trillion won in 2020 to 103 trillion won in 2050.
However, experts agree that if cognitive decline is detected and appropriately treated at the earlier stage of ‘mild cognitive impairment’ rather than at the severe dementia stage that significantly impairs daily life, rapid worsening of the disease can be prevented.
DTx Treating Dementia at the Pre-Dementia Stage
For this reason, companies are competing to develop digital therapeutics (DTx) that enable proper screening, classification, and treatment at the mild cognitive impairment stage. Representative companies currently developing related DTx in South Korea include Rowan, Hi, and Imocog.
Rowan’s ‘SuperBrain’ is a cognitive intervention therapy program aimed at preventing dementia in elderly individuals with dementia risk factors through cognitive training, exercise management, nutrition management, vascular management, and motivation enhancement. By continuing cognitive training in a game format, it strengthens nerve cells and provides exercise programs to activate brain cell functions, offering comprehensive management. Clinical trials have recognized actual effects such as significant thickening of the cerebral cortex compared to control groups.
Imocog is also developing a DTx called ‘Cogthera’ for patients with mild cognitive impairment. This mobile application-based therapeutic tool uses AI to adjust the difficulty of cognitive therapy tailored to individuals and aims to increase convenience by enabling cognitive training without human intervention.
Diagnosis and Screening: "Need for Insurance Coverage"
Competition to develop programs for dementia diagnosis and screening through digital means is intensifying. Imocog is currently developing the dementia diagnosis program ‘CogScreen’ and the digital neuropsychological test ‘Cognosis’.
Hi has embarked on developing the early dementia screening program ‘AlzGuard.’ It utilizes ‘digital biomarkers’ such as voice and eye movement to enable self-diagnosis of mild cognitive impairment. Currently, it can screen early dementia patients with nearly 90% accuracy. Alongside this, Hi has developed and is providing the cognitive enhancement chatbot ‘Samitalk’ service.
Meanwhile, overseas development of dementia-related DTx is not progressing well. Among the members of the Digital Therapeutics Alliance (DTA), a global coalition of DTx developers, Imocog is almost the only company developing DTx targeting dementia and mild cognitive impairment.
‘DTHR-ALZ,’ developed by U.S.-based Dthera Science, gained attention as a pure DTx without drug treatment and was designated as a breakthrough device by the U.S. FDA in August 2018. However, it ultimately faced failure when it ran out of capital and ceased operations in 2019. Ed Cox, former CEO of Dthera Science, pointed to delayed insurance reimbursement amid failures of candidate drugs linked to DTx as a major cause of failure.
One reason why insurance listing of DTx is considered a key factor for market growth in both dementia and the DTx industry is clear. Professor Hojin Choi of Hanyang University Guri Hospital’s Department of Neurology emphasized, “To ensure dementia patients can receive appropriate medical services, insurance coverage for cognitive intervention therapy is necessary,” adding, “Securing evidence for the therapeutic action is ultimately the top priority for inclusion in insurance coverage.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.