Estimated 80.3% Prevention Effect in Clinical Trial for Ages 6 Months to 4 Years
Health Authorities "Will Monitor Overseas Situations and Consult Relevant Ministries if Necessary"
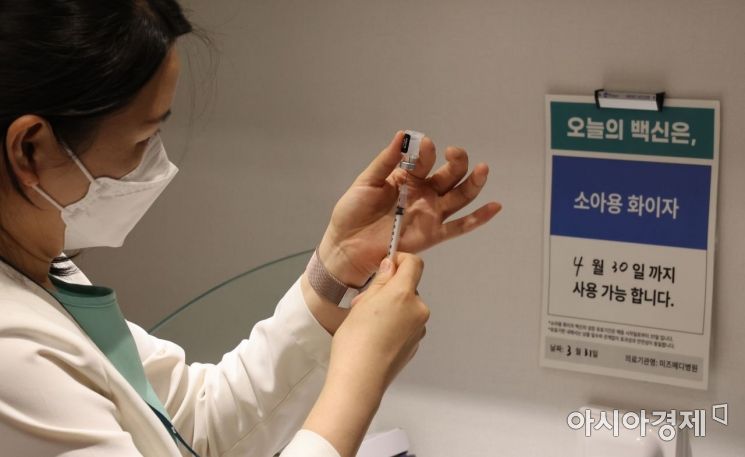 On March 31, when COVID-19 vaccinations for children aged 5 to 11 began, medical staff were preparing vaccines at the Department of Pediatrics and Adolescents at Mizmedi Hospital in Gangseo-gu, Seoul. Photo by Joint Press Corps
On March 31, when COVID-19 vaccinations for children aged 5 to 11 began, medical staff were preparing vaccines at the Department of Pediatrics and Adolescents at Mizmedi Hospital in Gangseo-gu, Seoul. Photo by Joint Press Corps
[Asia Economy Reporter Kim Jeong-wan] On the 23rd (local time), U.S. pharmaceutical company Pfizer announced that its COVID-19 vaccine has been confirmed effective for children under 5 years old through clinical trials. In response, health authorities are monitoring overseas trends regarding COVID-19 vaccination for children under 5 and stated that if necessary, they will consult with relevant government departments to review whether to proceed with vaccinations.
According to AP, AFP, and other news agencies, Pfizer confirmed through clinical trials that its COVID-19 vaccine is effective for children under 5 years old and plans to request emergency use authorization from the U.S. Food and Drug Administration (FDA) for the COVID-19 vaccine for children aged 6 months to 4 years.
Pfizer reported that after administering three doses of a 3μg vaccine to 1,678 children aged 6 months to 4 years, antibody levels met the FDA's required standards. The estimated COVID-19 prevention efficacy for the three-dose group was 80.3%.
Another U.S. pharmaceutical company, Moderna, requested emergency use authorization for its vaccine for children under 5 years old at the end of last month.
Meanwhile, on the 24th, health authorities stated regarding the promotion of COVID-19 vaccination for children under 5, "We will monitor overseas situations and, if necessary, consult with relevant departments such as the Ministry of Food and Drug Safety to review the matter."
Kwon Geun-yong, head of the COVID-19 Vaccination Management Team at the COVID-19 Vaccination Response Promotion Group, said at the regular briefing of the Central Disease Control Headquarters that "We are closely monitoring the vaccine approval status for children under 5 overseas" and made the above remarks.
Team leader Kwon said, "Decisions to expand the age range for COVID-19 vaccination have been made by comprehensively reviewing the epidemic situation and severity rate in the relevant age group, vaccine effectiveness, safety, and overseas trends," adding, "Vaccination for children under 5 will also be reviewed similarly by monitoring related situations and, if necessary, consulting with relevant departments such as the Ministry of Food and Drug Safety."
Health authorities also stated that they are continuously monitoring vaccine utilization plans in preparation for a possible resurgence that could begin as early as this summer. Team leader Kwon emphasized, "We will be able to judge what variants may emerge and the scale and season of any resurgence through additional information in the future," adding, "We will continue to monitor the situation and make every effort to prevent resurgence by maximizing the use of current vaccines and those under development."
He also said, "The vaccines currently administered have been confirmed to be more effective in preventing severe illness or death rather than infection during the Omicron wave," and "We continue to encourage the fourth dose, especially for the elderly."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














