[Asia Economy Reporter Kim Daehyun] The Ministry of Food and Drug Safety (MFDS) has decided to grant emergency use authorization for Pfizer's oral COVID-19 treatment, Paxlovid.
On the 27th, the MFDS stated "Considering the increasing number of confirmed COVID-19 cases and critically ill patients, the necessity of introducing an oral treatment that patients can take themselves, the MFDS's review of safety and efficacy, and the results of expert advisory meetings, the decision was made after deliberation by the 'Public Health Crisis Response Medical Product Safety Management and Supply Committee."
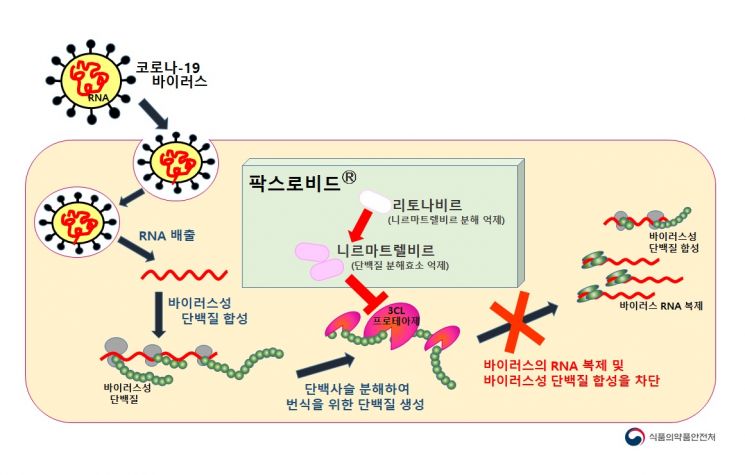
Paxlovid is the first oral treatment introduced in Korea. It is a drug that inhibits viral replication by blocking the protein-degrading enzyme (3CL protease), preventing the production of proteins necessary for virus replication, and is expected to help prevent patients admitted to residential treatment centers or undergoing home treatment from worsening to severe conditions.
The target patients are adults and children (aged 12 and older, weighing 40 kg or more) with mild to moderate COVID-19 who are at high risk of progressing to severe disease due to age, underlying conditions, etc. The treatment consists of taking two tablets of nirmatrelvir and one tablet of ritonavir twice daily (every 12 hours) for five days, and it should be administered as soon as possible within five days after symptom onset following a positive COVID-19 diagnosis.
Earlier, the Korea Disease Control and Prevention Agency (KDCA) requested emergency use authorization for Paxlovid from the MFDS on the 22nd, as the need for oral COVID-19 treatments increased during the phased return to normal life. Emergency use authorization is a system that allows manufacturers or importers to supply medical products not yet approved domestically in response to public health crises such as infectious disease pandemics.
An MFDS official stated, "Even after this emergency use authorization, we will intensify efforts to collect information on side effects and implement additional safety measures during use," adding, "By continuously analyzing and evaluating safety information, we will promptly provide precautionary guidance, suspend use, recall products, and take other necessary safety actions. If adverse effects occur, we will assess causality and provide compensation, prioritizing patient safety in all measures."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














