Institute for Basic Science Achieves Synthesis of Single-Atom Dimer Structure
Enables Production of Cobalt+Nickel Catalysts Four Times Cheaper Than Expensive Platinum
Revolutionary Economic Improvement in Water-Based Hydrogen Production Method
 Schematic diagram of the hydrogen generation process using NiCo-SAD. Image courtesy of the Institute for Basic Science.
Schematic diagram of the hydrogen generation process using NiCo-SAD. Image courtesy of the Institute for Basic Science.
[Asia Economy Reporter Kim Bong-su] Domestic researchers have developed a hydrogen production catalyst that is four times cheaper than existing ones. This development is attracting attention as a potential breakthrough in the hydrogen production method, which is one of the major energy alternatives in a decarbonized society.
The Korea Institute of Basic Science announced on the 22nd that the research team led by Deputy Director Lee Hyo-young of the Nanostructure Physics Research Division synthesized two single atoms to develop a single metal atom dimer catalyst that is four times cheaper than existing catalysts. It is characterized by the ability to produce hydrogen in any type of water and operate for a longer time compared to existing catalysts. It is expected to present a new direction for hydrogen production methods by enabling the production of commercial high-purity hydrogen cheaply and in an environmentally friendly way.
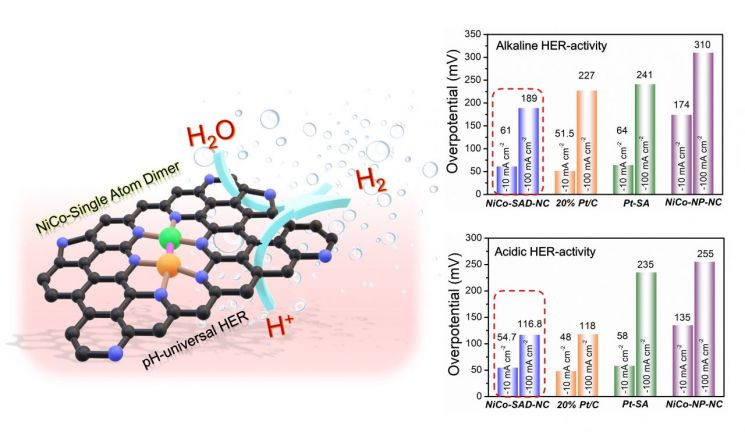
Hydrogen, which is gaining attention as an eco-friendly future fuel, can be obtained from water, leading to active research in this area. The only hydrogen production method that does not emit carbon dioxide is water electrolysis, which produces hydrogen by electrolyzing water. Although environmentally friendly, reducing production costs is crucial. This is because catalysts that improve production efficiency are still expensive. Precious metal platinum (Pt) catalysts can produce hydrogen regardless of the water’s properties such as acidity or alkalinity, but since the material is platinum, the unit price is high and there are limitations in stability during long-term operation.
The research team overcame these limitations by implementing dimers using inexpensive transition metals cobalt and nickel. They stably synthesized single atoms in a nickel-cobalt dimer structure on nitrogen-doped carbon supports that can be used in all types of water. The researchers found that while nickel and cobalt single atoms individually showed low hydrogen evolution activity, the dimer structure formed by combining the two single atoms produced a synergistic effect that maximized hydrogen production. They also synthesized other dimers such as cobalt-manganese and cobalt-iron using the same method.
The team confirmed that the single-atom nickel-cobalt dimer electrocatalyst can produce hydrogen at voltages similar to platinum catalysts. Furthermore, unlike conventional platinum catalysts which are stable for only 24 hours, the single-atom nickel-cobalt dimer was found to be usable for 50 hours without structural changes.
This research is significant in that it successfully realized the synthesis of a single-atom dimer structure, a long-standing challenge in the field of single atoms. By realizing a structure that previously existed only theoretically, they developed a new catalyst that is inexpensive yet possesses the advantages of precious metal platinum catalysts. It is expected to contribute to environmentally friendly hydrogen production by replacing expensive platinum catalysts.
Deputy Director Lee said, "By developing a low-cost, high-efficiency hydrogen production electrolysis catalyst, we have taken a step closer to an eco-friendly hydrogen production economy with zero carbon dioxide emissions."
This research was published online on the 19th in the international journal Nature Communications (IF 14.919).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














