[Asia Economy Reporter Cho Hyun-ui] On the 11th (local time), the European Medicines Agency (EMA) recommended approval for two COVID-19 antibody treatments, including Celltrion's Regkirona.
The Committee for Medicinal Products for Human Use (CHMP) under the EMA recommended approval for Regkirona to be used in treating patients infected with COVID-19 who are at high risk of progressing to severe disease. The treatment target is adult patients who do not require supplemental oxygen.
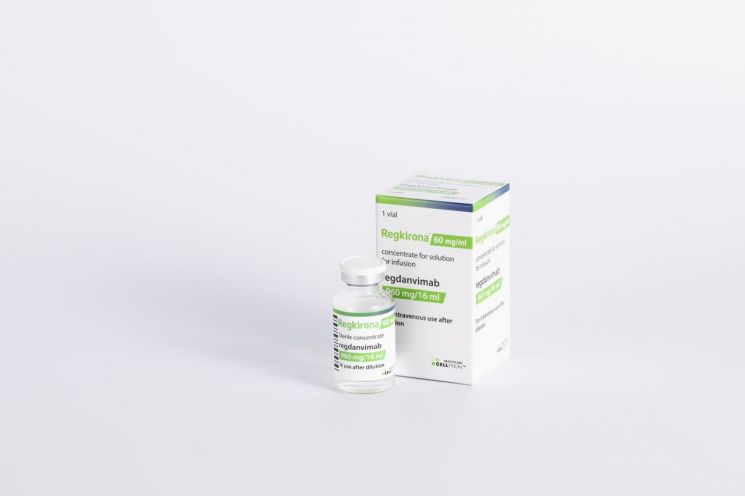
Regkirona is an intravenous COVID-19 antibody treatment developed by Celltrion, which received conditional approval from the Ministry of Food and Drug Safety in February. Subsequently, in September, it received full marketing authorization after expanding the range of eligible patients for administration.
The antibody treatment 'Ronapreve' by the U.S. pharmaceutical company Regeneron was also recommended for use in patients aged 12 and older who are at high risk of progressing to severe disease.
Ronapreve is the treatment that former U.S. President Donald Trump praised highly after receiving hospitalization treatment for COVID-19 and returning to the White House office.
The CHMP also added that Ronapreve can be used for the prevention of COVID-19 in adults aged 12 and older.
This is the first time that approval has been recommended for monoclonal antibody treatments in Europe. These treatments will be used for the first time in Europe, where COVID-19 is spreading at the fastest rate ever recorded.
Celltrion and Regeneron applied for marketing authorization from the EMA in early last month. However, the U.S. pharmaceutical company Eli Lilly withdrew its conditional approval application for its antibody treatment submitted to the EU last week, citing lack of demand.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














