KAIST Professor Min-Ki Choi's Team Publishes Related Paper in International Journal on May 17
[Asia Economy Reporter Kim Bong-su] A new concept of high-performance industrial catalyst capable of selectively converting only the desired reactants, similar to natural enzymes, has been developed. It is expected to enhance both economic efficiency and eco-friendliness in petrochemical processes.
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 9th that Professor Min-ki Choi's research team from the Department of Bio and Chemical Engineering recently achieved this research result and published a related paper in the online edition of the international journal 'Angewandte Chemie' on the 17th of last month.
Catalysts are substances used in most petrochemical processes, from basic hydrocarbon production to the manufacture of various chemical products. Developing catalysts with high selectivity that produce only the desired products is essential to improve the economic efficiency and eco-friendliness of these processes.
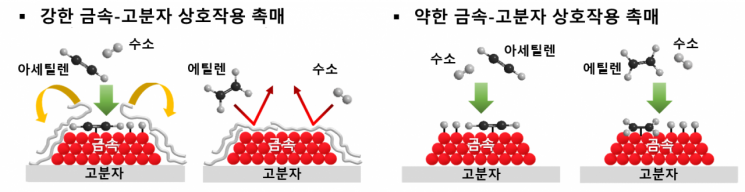
Among catalysts existing on Earth, enzymes exhibit the highest selectivity. Enzymes are natural polymers made of proteins that have a three-dimensional structure surrounding the active site where reactions occur. Depending on the protein structure and interactions with the active site, enzymes regulate access so that only specific reactants can selectively approach, resulting in high selectivity. In this study, the research team proposed a new concept of catalyst design by using polymers similar to enzyme proteins to control interactions with metal active sites.
Polymers are macromolecules with high molecular weight formed by repetitive chemical bonds of certain monomers, and their functional groups can be easily adjusted depending on the monomers used in synthesis. The research team synthesized polymers containing functional groups capable of interacting with metals and created catalysts including palladium metal particles. Polymers that strongly interact with metals exhibited a three-dimensional structure surrounding the metal similar to enzymes, while polymers with weak interactions failed to surround the metal, leaving the metal surface exposed.
The research team applied the synthesized catalyst to the partial hydrogenation reaction of acetylene, which is very important in the ethylene production process in petrochemicals. Ethylene is a fundamental core raw material used to make various products such as plastics, vinyl, and adhesives, and in South Korea, it is mainly produced by cracking naphtha.
Ethylene produced in naphtha cracking facilities contains trace amounts of acetylene impurities. Since acetylene critically affects catalysts used in chemical product manufacturing, a hydrogenation process to remove it is essential. The key in this process is to selectively remove less than 1% acetylene without consuming more than 99% of ethylene.
Using the newly developed catalyst, the polymer selectively approached only acetylene, showing high selectivity. However, catalysts with polymers that weakly interacted and failed to cover the metal surface allowed access to both acetylene and ethylene, resulting in low selectivity. The stronger the polymer-metal interaction, the more it prevented the formation of coke, a carbon deposit causing deactivation, and suppressed metal particle agglomeration, maintaining high activity and selectivity even during long-term reactions.
Professor Min-ki Choi said, "The catalyst design method that mimics the principles of natural enzymes by controlling interactions between polymers and metals to selectively convert only desired reactants while possessing excellent stability is a new concept not previously reported worldwide," adding, "It is expected to be widely applied and utilized in various chemical reactions requiring high selectivity in the future."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














