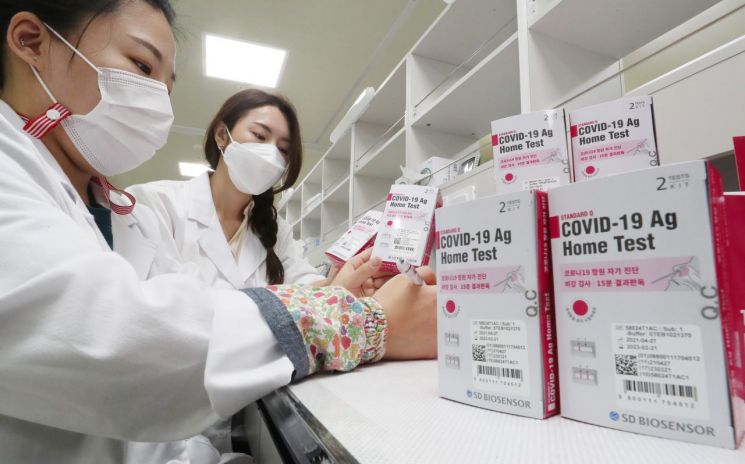
 At the SD Biosensor factory in Osong, Chungbuk, which develops and manufactures diagnostic kits that confirm COVID-19 infection using the real-time polymerase chain reaction (RT-PCR) method, final inspections were conducted on the 29th of last month in preparation for sales through pharmacies and the internet. [Image source=Yonhap News]
At the SD Biosensor factory in Osong, Chungbuk, which develops and manufactures diagnostic kits that confirm COVID-19 infection using the real-time polymerase chain reaction (RT-PCR) method, final inspections were conducted on the 29th of last month in preparation for sales through pharmacies and the internet. [Image source=Yonhap News]
[Asia Economy Reporter Lee Chun-hee] Thanks to the development of COVID-19 diagnostic kits and other COVID-19 quarantine supplies, the number of approvals for domestically manufactured medical devices surpassed that of imported medical devices for the first time since 2011.
The Ministry of Food and Drug Safety (MFDS) published the "2020 Medical Device Approval Report" on the 31st, which contains the status of medical device approvals, certifications, and notifications from last year.
Last year, the total number of medical device product approvals (including certifications and notifications) was 8,183, similar to the 8,269 cases in 2019. In particular, domestically manufactured medical devices accounted for 4,222 cases, representing 51.6% of the total, surpassing imported medical devices for the first time since 2011.
The MFDS analyzed this change, stating, "It appears to be influenced by the increased development of COVID-19 quarantine supplies such as COVID-19 diagnostic reagents." According to the MFDS, a total of 13 COVID-19 diagnostic reagents were approved last year (▲9 gene amplification (PCR) ▲2 antigen ▲2 antibody), and a total of 236 export approvals were granted. This accounts for 21.9% of the total 1,132 medical device manufacturing approvals last year. Along with this, the number of certifications and notifications for 'skin infrared thermometers' and 'specimen collection tools' used in COVID-19 quarantine also sharply increased last year.
The development of artificial intelligence (AI)-based medical devices also surged. AI-based medical devices received 50 approvals last year, a fivefold increase compared to 10 in 2019. Notably, 45 of these were domestically manufactured, accounting for 90%. It was found that AI-based software medical devices, mainly used to analyze medical images such as X-rays for diagnosis, were developed.
With growing interest in health recently, the development of personal medical devices was also active. Developments included 393 cases of eyeglass lenses, 130 hearing aids, 43 contact lenses, and 40 thermometers.
The number of approvals for converged medical devices such as tissue repair biomaterials, stents, and non-implantable vascular access devices also showed a continuous increase, reaching 23 cases last year since 2018.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














