Dr. Ryu Juhee's Team at KIST Explores Trillion-Won Market Replacement Potential
 Cancer treatment paradigm is changing. Reference photo.
Cancer treatment paradigm is changing. Reference photo.
[Asia Economy Reporter Kim Bong-su] A new anticancer therapeutic agent that improves existing anticancer drugs worth about 1 trillion KRW on the market by eliminating side effects and enhancing efficacy has been developed by domestic researchers. Since it utilizes existing substances, clinical trials and commercialization are expected to be much easier, drawing attention.
The Korea Institute of Science and Technology (KIST) announced on the 30th that Dr. Ryu Ju-hee (pictured below) and her research team at the Theragnosis Research Center have developed an anticancer prodrug that minimizes toxicity to normal cells including immune cells, selectively targets and kills cancer cells, and enhances the patient’s immune status to aid anticancer immunotherapy.
Unlike conventional cancer treatments that directly attack and kill cancer cells, anticancer immunotherapy strengthens the body's immune system to kill cancer cells and has recently gained attention as a new cancer treatment method. However, it is only effective in about 20% of patients with good baseline immunity, limiting its application to the majority of patients.
In particular, doxorubicin, a drug used in hospitals for cancer treatment, has recently been found to enhance patients’ immunity due to various substances released when cancer cells die. However, doxorubicin can cause toxicity and inflammatory responses not only to cancer cells but also to normal cells, and especially shows toxicity to immune cells, which can actually reduce the patient’s immune level.
The center had already developed an anticancer drug last year that suppresses resistance to doxorubicin and selectively kills cancer cells without reacting to normal cells. Building on the finding that doxorubicin can enhance patients’ immune capacity, the research team developed a drug that can be used for anticancer immunotherapy.

The developed drug is conjugated with a peptide that inactivates doxorubicin, rendering it inactive and non-toxic until activated by enzymes abundantly present in cancer cells, thereby exhibiting anticancer efficacy. Therefore, it is not activated in normal cells, preventing toxicity and inflammatory responses, but is activated in cancer cells, killing them through doxorubicin components and enhancing the patient’s immune capacity, thus inducing a robust anticancer immune response in the future.
As a result, the developed anticancer drug significantly enhanced anticancer immune responsiveness in nonclinical animal models and greatly reduced side effects such as inflammatory responses and toxicity in normal tissues. It is expected that the drug can be administered at higher concentrations without concerns about side effects to improve anticancer treatment efficacy.
Since the drug was developed using an existing drug already used in clinical settings, clinical trials are relatively straightforward, simplifying commercialization procedures. The simple chemical structure combining four amino acids and doxorubicin makes the manufacturing process simple and facilitates mass production. Therefore, it has significant advantages in drug manufacturing considering clinical and commercial applications.
Dr. Ryu Ju-hee said, “For the majority of patients to benefit from the remarkable therapeutic effects of immunotherapy, their immune levels must be appropriately elevated. Anticancer prodrugs that maintain anticancer immune responses while reducing toxicity and inflammatory responses in normal tissues can make a significant contribution.”
This research was published in the latest issue of the international journal in the field of materials and biomaterials, ‘Biomaterials’ (IF:10.317, top 2.6% in JCR category).
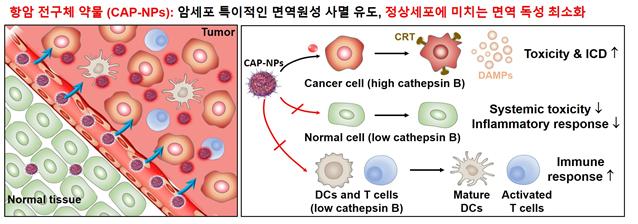 Anticancer prodrug accumulates in tumor tissue through the Enhanced Permeability and Retention (EPR) effect and is specifically activated by cathepsin B enzyme, which is overexpressed in cancer cells, releasing doxorubicin. It induces tumor-specific immunogenic cell death within the tumor tissue while minimizing toxicity to immune cells, leading to effective maturation and activation of T cells and other immune cells within the tumor. At the same time, it significantly reduces systemic toxicity and inflammatory responses in normal organs. Image provided by KIST
Anticancer prodrug accumulates in tumor tissue through the Enhanced Permeability and Retention (EPR) effect and is specifically activated by cathepsin B enzyme, which is overexpressed in cancer cells, releasing doxorubicin. It induces tumor-specific immunogenic cell death within the tumor tissue while minimizing toxicity to immune cells, leading to effective maturation and activation of T cells and other immune cells within the tumor. At the same time, it significantly reduces systemic toxicity and inflammatory responses in normal organs. Image provided by KIST
◇ Research Background
Anticancer immunotherapy, which has attracted great interest, shows remarkable effects, but the number of patients who respond is limited. It is known that cancers with good intrinsic immune capacity (hot tumors) respond better than those without (cold tumors). Therefore, enhancing the intrinsic immune capacity of cancer is considered very important in anticancer immunotherapy, and chemotherapeutic agents like doxorubicin, which can induce immunogenic cell death of cancer cells, have recently gained renewed attention. However, doxorubicin can cause toxicity and inflammatory responses in normal cells and especially toxicity to immune cells, making intrinsic immune formation difficult. Accordingly, the research team developed an anticancer prodrug that effectively induces immunogenic cell death in cancer cells while not causing toxicity or inflammatory responses in normal cells.
◇ Research Content
The cancer cell-specific anticancer prodrug was formed into stable nanoparticles by conjugating the existing anticancer drug doxorubicin with a peptide that can be specifically cleaved by cathepsin B enzyme, which is overexpressed in cancer cells. The anticancer prodrug is activated to doxorubicin in cancer cells, inducing immunogenic cell death at levels similar to doxorubicin, converting cancers with low baseline immunity into hot tumors, as confirmed by cell and mouse preclinical experiments. However, in normal cells, it remains inactive and does not induce immunogenic cell death, resulting in reduced inflammatory responses in normal tissues. Expression of the inflammation-related receptor Toll-like receptor 4 (TLR-4) decreased, and levels of TNF-α and IL-6 in blood were lowered. In combination therapy with the immunotherapeutic PD-L1 antibody, the anticancer prodrug showed strong tumor suppression and high survival rates.
The research team demonstrated through cell and mouse preclinical experiments that the cancer cell-specific anticancer prodrug enables not only conventional chemotherapy but also anticancer immunotherapy via induction of immunogenic cell death. The low toxicity and inflammatory induction in normal cells alleviated concerns about immune-related side effects. The prodrug shows potential not only as a monotherapy but also as a combination therapy with various immune checkpoint inhibitors.
 Cancer cells / Photo by Asia Economy DB
Cancer cells / Photo by Asia Economy DB
Below is a Q&A on the research results.
◇ What motivated or was the background for starting this research?
Doxorubicin, a widely used chemotherapeutic anticancer drug, is known to enable anticancer immunotherapy by inducing immunogenic cell death. However, its use is limited due to side effects caused by inflammatory responses in normal cells. Additionally, although various immunotherapies have been developed, their high cost makes it difficult for cancer patients to access them, which was also a motivation for this research.
◇ What is different about this achievement?
The anticancer prodrug directly demonstrated the inhibition of inflammatory responses in normal tissues compared to conventional drugs through data showing decreased expression of inflammation-related receptors such as Toll-like receptor 4 and reduced levels of TNF-α and IL-6 in blood. In other words, the cancer cell-specific anticancer prodrug induces immunogenic cell death in cancer cells to trigger anticancer immunotherapy while reducing immune-related side effects in normal cells.
◇ How could it be utilized if commercialized?
Doxorubicin, the active component of the anticancer prodrug, is already used clinically for about 18 types of cancer. The low toxicity and inflammatory response of the prodrug in normal tissues allow for higher dosing, expanding its potential use to various cancer types. It also has advantages such as relatively easy mass production and very low manufacturing costs, increasing its clinical applicability.
◇ What are the expected effects and challenges for commercialization?
DOXIL®, a liposomal nanoformulation of doxorubicin and a competitor technology, is a blockbuster drug with sales around 1 trillion KRW. Many researchers have attempted to develop anticancer drugs to replace it, but no alternative drug suitable for clinical use has been developed due to various technical and industrial challenges. If the prodrug’s technological superiority can be applied clinically, it is expected to replace the existing doxorubicin market worth 1 trillion KRW. This requires consideration not only of technical aspects such as efficacy and toxicity but also industrial factors that may arise during production and clinical application.
 Cancer patients in Gwangju and Jeonnam are increasing. In particular, colorectal cancer has been rapidly rising recently. Medical staff performing colorectal cancer surgery.
Cancer patients in Gwangju and Jeonnam are increasing. In particular, colorectal cancer has been rapidly rising recently. Medical staff performing colorectal cancer surgery.
◇ Terminology Explanation
1. Cathepsin B
Cathepsin B is an acidic protease present in lysosomes. It is mainly involved in cell migration, differentiation, proliferation, and metastasis. Especially in cancer cells where these processes are active, cathepsin B expression is known to be relatively much higher than in normal cells.
2. Immunogenic Cell Death (ICD)
Immunogenic cell death is a form of cell death that activates the immune system during cell death, inducing effective immune stimulation through activation of antigen-presenting cells such as dendritic cells and subsequent activation of specific T cell responses.
3. Hot Tumor
The tumor microenvironment is classified as cold or hot tumor based on the degree of immune T cell infiltration. Hot tumors, which have sufficient immune T cells in the tumor microenvironment, contrast with cold tumors that lack immune cell infiltration. Hot tumors show relatively higher response rates to immune checkpoint inhibitors, and research is ongoing to convert cold tumors into hot tumors to improve immunotherapy response rates.
4. Toll-like Receptor 4 (TLR-4)
Toll-like receptor 4 is known as a key factor activating innate immunity, but its upregulation in macrophages by doxorubicin is an important step in generating toxic side effects. Increased expression of TLR-4 in macrophages by doxorubicin promotes secretion of pro-inflammatory cytokines.
5. Immune Checkpoint Inhibitor PD-L1 Antagonist
Immune checkpoint inhibitors refer to various inhibitory pathways that play important roles in maintaining self-tolerance and regulating immune responses. Tumor cells exploit immune checkpoints to evade immune cell attacks. PD-L1 antibodies, known as immune checkpoint antagonists, bind to PD-L1 receptors on tumor cell membranes to induce T cell attacks. Drugs such as Nivolumab and Pembrolizumab have been approved by the FDA and are used in combination immunotherapy.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










