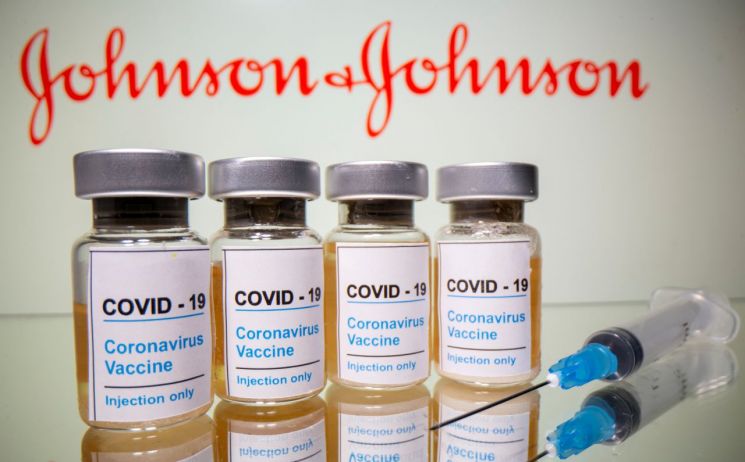
[Asia Economy Reporter Suyeon Woo] On the 13th (local time), U.S. health authorities recommended suspending the use of the Johnson & Johnson (J&J) COVID-19 vaccine as they are reviewing cases of thrombosis occurring after vaccination.
The U.S. Centers for Disease Control and Prevention (CDC) and the Food and Drug Administration (FDA) stated in a joint statement that they are reviewing six cases of a "rare but serious" form of thrombosis in individuals vaccinated with the J&J vaccine and recommended suspending its use.
As of the previous day, 6.8 million doses of the J&J vaccine had been administered in the U.S. The CDC plans to review the problematic cases and assess their potential implications at the Advisory Committee on Immunization Practices meeting on the 14th, while the FDA will analyze the CDC's evaluation and conduct individual case investigations concurrently.
The FDA said, "We recommend suspending the use of the J&J vaccine until the review process is complete," adding, "Safety related to the vaccine is our top priority, and we will take all cases of health abnormalities following vaccination seriously."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














