Trillion-Won Annual Sales Giant Market
Patents Expiring One After Another by 2025
Competition in the Stelara Biosimilar Market
Among Celltrion, Dong-A ST, and Others
The pharmaceutical and bio industries are becoming increasingly active as blockbuster biopharmaceutical patents approach expiration. They are accelerating the development of biosimilars to capture the massive market worth trillions of won before the wave of substance patents expires by 2025. Fierce competition is expected among leading domestic biosimilar companies such as Celltrion and Samsung Bioepis, as well as Dong-A ST, Samchundang Pharm, and Alteogen, over major markets including the psoriasis treatment 'Stelara', the macular degeneration treatment 'Eylea', and the skeletal disease treatment 'Prolia'.
An industry insider said, "Biosimilars have less burden in terms of development time and cost compared to new drugs," adding, "With successful cases like Celltrion's Remsima, pharmaceutical companies and bio ventures are increasingly targeting the biosimilar markets of drugs whose patents will expire within 3 to 4 years as their next growth engine."
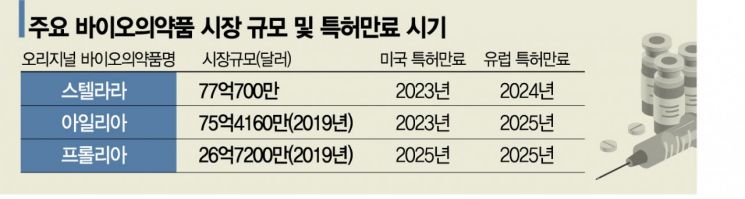
◆ 8 trillion won Stelara market, Dong-A ST joins the race = According to the industry on the 8th, among domestic companies, Celltrion, Samsung Bioepis, and Dong-A ST are developing biosimilars of Stelara. Stelara, developed by Janssen, is an autoimmune disease treatment for psoriasis and Crohn's disease, with global annual sales reaching $7.707 billion (about 8.7 trillion won) last year. Its patents in the U.S. and Europe expire consecutively in 2023 and 2024.
Leading biosimilar powerhouse Celltrion began global Phase 3 trials for the Stelara biosimilar ‘CT-P43’ at the end of last year. In January, it also received approval from the Korean Ministry of Food and Drug Safety for domestic Phase 3 trials. Celltrion plans to complete Phase 3 trials by the first half of 2023.
Dong-A ST has chosen Stelara as its second biosimilar challenge following the anemia treatment Aranesp, signaling a direct confrontation with Celltrion. In January, Dong-A ST received FDA approval for Phase 3 clinical trials of ‘DMB-3115’ and plans to start the trials within this month. Recently, Samsung Bioepis also joined the competition by initiating Phase 1 clinical trials of ‘SB17’ in France.
◆ Eylea, four domestic companies in fierce competition = The biosimilar market for ‘Eylea’, an ophthalmic disease treatment jointly developed by Regeneron in the U.S. and Bayer in Germany, has also heated up. Eylea is used to treat various eye diseases such as macular degeneration and macular edema, with patents in major global markets expiring by 2025, starting with Japan and China next year. Given its massive global annual sales of $7.5416 billion (about 8.5 trillion won, based on 2019), Celltrion, Samsung Bioepis, Samchundang Pharm, and Alteogen have all jumped into development domestically.
Samsung Bioepis is the fastest in development. Since July last year, it has been conducting global Phase 3 trials of ‘SB15’, planning to complete comparative studies on efficacy, safety, pharmacokinetics, and immunogenicity between SB15 and the original drug with 446 macular degeneration patients by February next year. Notably, Samsung Bioepis has also completed development of a biosimilar for Lucentis, which, along with Eylea, accounts for over 80% of the global macular degeneration treatment market. Celltrion started Phase 3 clinical trials for the Eylea biosimilar ‘CT-P42’ last month. By the second half of next year, it will conduct comparative studies on efficacy and safety between CT-P42 and Eylea with diabetic macular edema patients across 13 countries.
Samchundang Pharm, which entered global Phase 3 trials last year, aims to complete Phase 3 and obtain product approval by next year, with plans to launch the product in 2023. Samchundang Pharm has secured formulation patents, enabling it to circumvent Eylea’s formulation patents. Alteogen recently completed domestic Phase 1 trials and is preparing to start global Phase 3 trials. Although it has not yet entered Phase 3, its possession of formulation patents is considered a strong advantage for faster market entry compared to competing biosimilars.
◆ "Despite red ocean concerns, competitiveness is sufficient" = The biosimilar market for Prolia, with annual sales of about 3 trillion won, is also attracting attention. In February, Celltrion received FDA approval for the Phase 3 clinical trial plan of ‘CT-P41’. It aims to complete Phase 3 trials by the first half of 2024 and commercialize the product in line with the expiration of U.S. substance patents in February 2025. CT-P41, along with biosimilar candidates for Eylea and Stelara, is expected to be a key part of Celltrion’s blueprint to obtain approval for at least one product annually until 2030.
Samsung Bioepis is also conducting clinical trials for ‘SB16’. Since the end of last year, it has accelerated development through an overlap strategy, conducting Phase 1 and Phase 3 trials with actual patients simultaneously.
Lee Seung-gyu, Vice Chairman of the Korea Bio Association, said, "With major original biopharmaceutical patents expiring by 2025, competition to become the first biosimilar is fierce," adding, "Although the entry of global pharmaceutical companies with production facilities may turn the market into a red ocean, domestic companies like Celltrion and Samsung Bioepis hold leadership in the biosimilar market with economically viable products."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















