Revealing the Secrets of the Biological Clock with Mathematical Models
Obesity, Dementia, and Aging as Causes of Insomnia
New Treatment Methods Expected for Sleep Disorders
 Various studies on sleep deprivation and increased activity time due to the implementation of daylight saving time have raised doubts about the effectiveness of the system, highlighting the decline in office workers' job performance and health issues. Photo by Getty Images
Various studies on sleep deprivation and increased activity time due to the implementation of daylight saving time have raised doubts about the effectiveness of the system, highlighting the decline in office workers' job performance and health issues. Photo by Getty Images
[Asia Economy Reporter Junho Hwang] The primary cause of our circadian rhythm, which governs our 24-hour daily cycle including sleep, has been uncovered by a domestic research team. Previously, it was known that PER proteins operate the biological clock by suppressing gene transcription in the cell nucleus, but the researchers went a step further to identify the mechanism by which PER proteins simultaneously penetrate the nucleus at the same time. Through this study, the team pointed out that obesity, dementia, and aging cause cytoplasmic congestion, which is a key factor leading to instability in the sleep cycle.
Unraveling the Secret of the 24-Hour Cycle with a Mathematical Model
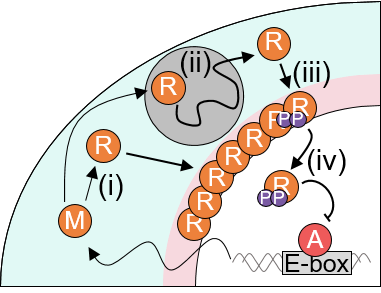 Movement and quantity of intracellular PER molecules over time simulated through a spatiotemporal probabilistic mathematical model
Movement and quantity of intracellular PER molecules over time simulated through a spatiotemporal probabilistic mathematical model
Professor Jaekyung Kim of the Department of Mathematical Sciences at the Korea Advanced Institute of Science and Technology (KAIST) announced on the 9th that he solved the enigma of the biological clock by developing a spatiotemporal stochastic model describing molecular movements within cells.
The 24-hour biological clock is known to be regulated by PER proteins accumulated in the cytoplasm for 12 hours, which then enter the nucleus to inhibit gene transcription, leading to a decrease in PER levels over the next 12 hours. However, it had not yet been revealed whether PER proteins produced at different times penetrate the nucleus simultaneously.
Using this model, the research team discovered that PER proteins must sufficiently condense around the nucleus to be phosphorylated simultaneously and enter the nucleus together. Professor Kim explained, "Thanks to the phosphorylation synchronization switch, thousands of PER proteins can enter the nucleus together at a consistent time, creating a stable circadian rhythm."
Obesity, Dementia, and Aging as Key Factors in Sleep Cycle Instability
Based on this, the team also identified the causes of unstable circadian rhythms and sleep cycles. When substances such as lipid droplets, which interfere with PER protein condensation around the nucleus, accumulate excessively in the cell, causing cytoplasmic congestion, the phosphorylation switch fails to operate, resulting in sleep disorders.
In collaboration with Professor Joo-Gon Lee of Florida State University, the research team validated these findings experimentally. They further demonstrated that obesity, dementia, and aging induce cytoplasmic congestion, which is a key factor causing instability in the sleep cycle.
Professor Jaekyung Kim stated, "This research reveals the causes of unstable sleep induced by obesity, dementia, and aging through the convergence of mathematics and life sciences." He added, "Resolving cytoplasmic congestion is crucial for treating sleep disorders," and explained, "This study is significant in that it presents a new paradigm for the treatment of sleep disorders."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














