Launch of the 'Bio SoBujang Solidarity Cooperation Council'
857 Billion Won Support Over 5 Years for Development of 16 Materials, Parts, and Equipment
Minister of Industry: "K-Bio Will Spread Through 'Solidarity and Cooperation'"
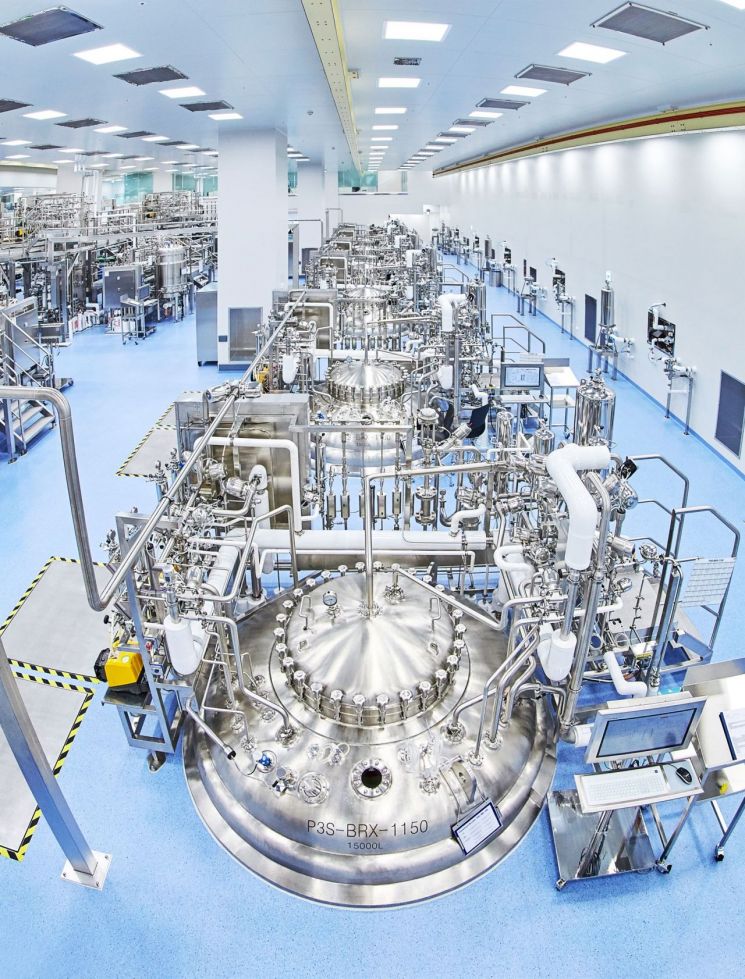 The bioreactor hall of Samsung Biologics Plant 3 located in Songdo International City, Incheon. (Photo by Samsung Biologics)
The bioreactor hall of Samsung Biologics Plant 3 located in Songdo International City, Incheon. (Photo by Samsung Biologics)
[Asia Economy Reporter Moon Chae-seok] A consultative body consisting of 55 bio companies, including Celltrion and Samsung Biologics, has been established. The government has decided to extend the inspection cycle for pressure vessels in the bio industry from 2 years to 4 years in the first half of next year to reduce the production order burden.
On the 24th, the Ministry of Trade, Industry and Energy announced the launch of the 'Bio Materials, Components, and Equipment Solidarity Cooperation Consultative Body' to strengthen the competitiveness of bio materials, components, and equipment.
A total of 55 companies are participating, including 13 bio materials, components, and equipment demand companies such as Celltrion and Samsung Biologics, and 42 supply companies such as Amicogen. The Korea Bio Association and the Korea Institute for Advancement of Technology support the operation of the consultative body.
The participating institutions will collaborate from the development of core bio materials, components, and equipment technologies to the development of items by supply companies that meet the requirements of demand companies. Demand companies plan to support demonstration tests and technical consultations.
The Ministry of Trade, Industry and Energy announced the 'Solidarity and Cooperation Industry Strategy Promotion Plan' on the occasion of the launch ceremony of the consultative body.
The consultative body was launched jointly by the private sector to promote qualitative soundness in Korea's biopharmaceutical industry.
The domestic biopharmaceutical production scale recorded 2.6002 trillion KRW last year, growing 16.6% compared to the previous year, but the localization rate of production equipment remained at about 16.5% as of May last year.
Through the solidarity and cooperation strategy, it is expected that bio demand companies will secure flexible and stable raw materials and equipment to strengthen production competitiveness.
Supply companies are also evaluated to have opportunities to enter the global market through core technology development and track record acquisition.
Meanwhile, during a preliminary discussion on the same day, the bio industry requested the government to extend the inspection cycle for pressure vessels in bio plants.
The industry explained that due to the characteristics of Good Manufacturing Practice (GMP) for pharmaceuticals, stopping equipment for open inspections takes 40 to 48 days to restart, which burdens production orders.
They argued that since the pressure and temperature in bio processes are mostly at atmospheric pressure and room temperature levels, the risk is not higher than in other industries. They appealed to consider that pressure vessels are being safely managed.
In response, Minister Sung Yun-mo of the Ministry of Trade, Industry and Energy promised to reform the system to extend the inspection cycle for pressure vessels in the bio industry from the current 2 years to 4 years in the first half of next year.
The ministry expects that if bio companies benefit from the inspection starting next year, the production competitiveness of Korea's bio industry will be further strengthened.
Additionally, the ministry plans to support 85.7 billion KRW over the next five years for the development of 16 materials, components, and equipment such as filters, media, and bioreactors.
Minister Sung said, "I consider it meaningful to launch the bio materials, components, and equipment consultative body as the first case of solidarity and cooperation," and emphasized, "When the value of 'solidarity and cooperation' is implanted in the bio industry, K-Bio will expand to a wider and higher level in the global market."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














