[Asia Economy Reporter Choi Dae-yeol] The government anticipates that the antibody treatment for the novel coronavirus infection (COVID-19) being developed by Celltrion could enter clinical trials overseas as early as next month. This treatment targets patients with severe symptoms, but the number of severe patients in South Korea is decreasing.
At a briefing on the 2nd, Kwon Jun-wook, Deputy Director of the Central Disease Control Headquarters, stated, "We have completed animal testing, and primate testing is next. We understand that negotiations are underway with European countries to conduct clinical trials abroad in July."
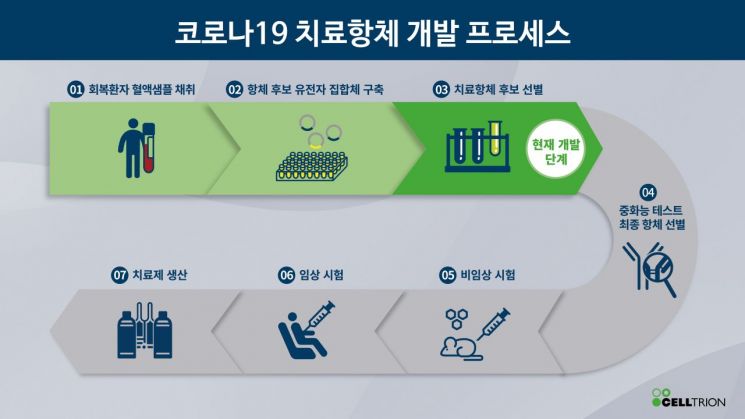
The government has been soliciting research projects for treatment and vaccine development as the COVID-19 situation spreads, conducting joint research with companies divided by field. Celltrion is responsible for the antibody treatment. This approach utilizes the human immune response and is a relatively fast-moving field, with companies like Lilly recently entering clinical trials overseas.
According to the Disease Control Headquarters, as of 0:00 this morning, there are 2 severe patients and 9 critical patients. These patients have difficulty or are unable to breathe on their own, but the number is insufficient to proceed with clinical trials. Previously, clinical trials planned with Gilead's remdesivir in the U.S. were also unable to proceed due to a lack of patients.
The treatments currently being pursued as emergency research projects by the government are broadly divided into four categories. Along with the antibody treatment being developed with Celltrion, there is also a plasma treatment being conducted through GC Green Cross. The plasma treatment uses antibodies formed in recovered patients, and among the ongoing research projects, the quarantine authorities expect this to be the first to become available.
Additionally, drug repurposing?applying drugs previously approved or developed for other purposes to different diseases?and new drug development are underway. In the case of remdesivir, after receiving emergency approval in the U.S., a decision on its introduction in South Korea is pending. Deputy Director Kwon said, "I am participating in a meeting held today by the Ministry of Food and Drug Safety to discuss this."
He added, "These treatments are for severe and critical patients, and currently, they are not expected to have antiviral effects that reduce transmission in the early stages of infection. While they will reduce fatality rates and complications, thereby lowering deaths, they will not stop the COVID-19 pandemic itself. Therefore, all countries are ultimately aiming to develop a vaccine."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.