Essential Industrial Material Hydrogen Peroxide for Semiconductor Cleaning
8-Fold Productivity Increase and Cost Reduced to 1/2000
Elucidation of Catalyst Activity Control Principle at Atomic Level
 The research team developed a new electrocatalyst structure with cobalt atoms placed on graphene. Using 1kg of this catalyst can produce 341.2kg of hydrogen peroxide per day. This performance is about eight times higher than that of existing expensive precious metal catalysts.
The research team developed a new electrocatalyst structure with cobalt atoms placed on graphene. Using 1kg of this catalyst can produce 341.2kg of hydrogen peroxide per day. This performance is about eight times higher than that of existing expensive precious metal catalysts.
[Asia Economy Reporter Junho Hwang] A catalyst that can increase the production efficiency of hydrogen peroxide, one of the key materials in the chemical and pharmaceutical industries such as semiconductor cleaning agents, by up to 8 times has been developed by a domestic research team. It is eco-friendly as it uses only oxygen and water, and the cost can be reduced to 1/2000, making it competitive as well.
The research team led by Taek-Hwan Hyun, head of the Nanoparticle Research Group at the Institute for Basic Science, and Young-Eun Sung, deputy head of the research group, in collaboration with Professor Jong-Seok Yoo’s team at the University of Seoul, announced on the 14th that they developed an electrocatalyst that can produce hydrogen peroxide in an eco-friendly way using only oxygen and water.
Producing Hydrogen Peroxide with Electrocatalyst
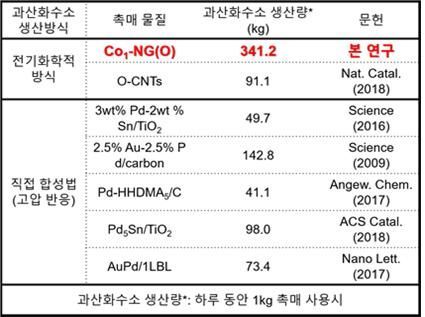 Comparison of Hydrogen Peroxide Production between the Catalyst Developed by the Research Team and Existing Catalysts
Comparison of Hydrogen Peroxide Production between the Catalyst Developed by the Research Team and Existing Catalysts
The research team devised a method to produce hydrogen peroxide by controlling catalytic activity at the atomic level. The catalyst developed by the researchers consists of cobalt (Co) atoms placed on two-dimensional graphene. When this catalyst is placed in an oxygen-saturated aqueous solution and electricity is applied, hydrogen peroxide can be produced without adding any other compounds.
This catalyst is cost-effective as it does not use precious metals such as platinum or palladium like conventional catalysts. As of December 24, cobalt costs about 37,000 KRW per kilogram, which is much cheaper than palladium (about 77 million KRW per kilogram).
This catalyst has about 8 times better production performance than existing catalysts. When using 1 kg of the catalyst, it can produce 341.2 kg of hydrogen peroxide per day. The catalyst was confirmed to maintain more than 98% of its initial performance even after conducting continuous hydrogen peroxide production experiments for over 110 hours.
In particular, the research team pointed out that this catalyst can be recovered and recycled after the chemical reaction, so no waste catalyst is generated, which is an environmentally friendly advantage.
Hydrogen peroxide is widely used not only in everyday products such as toothpaste and kitchen detergents but also in medical settings requiring sterilization, wastewater treatment agents, and semiconductor processes that require impurity removal. Until now, industrial hydrogen peroxide has mainly been produced by the anthraquinone process. However, this process consumes a lot of energy and has been criticized for producing by-products that cause environmental pollution.
Controlling Catalytic Activity at the Atomic Level
 Hyun Taek-hwan, Head of the Nanoparticle Research Group (left), Sung Young-eun, Deputy Head of the Research Group
Hyun Taek-hwan, Head of the Nanoparticle Research Group (left), Sung Young-eun, Deputy Head of the Research Group
The research team emphasized that they were the first in the world to identify the principle that can enhance the activity of heterogeneous catalysts at the atomic level. Since this catalyst can synthesize products stably and environmentally friendly at room temperature and atmospheric pressure, it is expected to be widely used in various chemical processes.
Deputy Head Young-Eun Sung said, "We got the idea for this research based on the study results that relatively inexpensive atoms such as iron, cobalt, and nickel, when stabilized on graphene, effectively mediate electrochemical reactions. We demonstrated that catalytic activity can be controlled at the atomic level and confirmed its validity through computational chemistry. By changing the structure around the cobalt atoms, we were able to develop a catalyst that shows world-class hydrogen peroxide production performance,” she explained.
Head Taek-Hwan Hyun said, "We have made it possible to produce hydrogen peroxide, one of the world’s top 100 industrial chemicals, in an environmentally friendly and economical way. We expect that this will improve productivity not only in hydrogen peroxide production but also in many chemical reactions using catalysts in the future."
Meanwhile, the research results were published on the 14th (Korean time) in Nature Materials, a world-renowned journal in the field of materials science.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














