Ministry of Health and Welfare and Ministry of Food and Drug Safety Launch “Immediate Market Entry Medical Technology” System
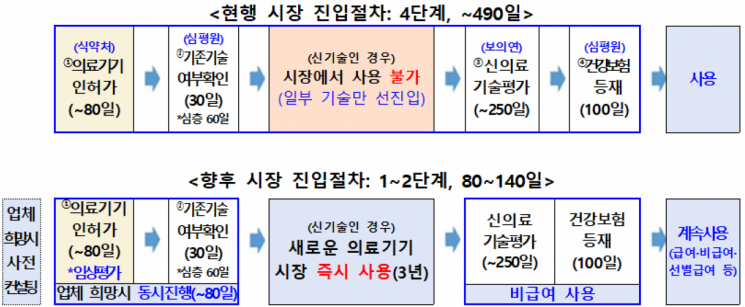
Procedures Reduced from Up to 490 Days to as Few as 80
Strict Oversight on Safety and Non-Reimbursed Use... “Immediate
Medical devices incorporating innovative technologies, such as artificial intelligence (AI) or medical robots, may now be introduced into clinical settings in as little as 80 days. This is expected to dramatically resolve the longstanding issue where complex administrative procedures delayed the market entry of outstanding technologies for over a year.
The Ministry of Health and Welfare and the Ministry of Food and Drug Safety announced that the "Immediate Market Entry Medical Technology" system will be fully implemented starting January 26. The core of this system is that if an innovative medical device receives approval from the Ministry of Food and Drug Safety after undergoing clinical evaluations that meet international standards, it can be used in medical settings immediately without a separate new medical technology assessment.
Previously, for a new medical device to be used in hospitals, it had to pass through four high hurdles: approval from the Ministry of Food and Drug Safety (80 days), confirmation of existing technology by the Health Insurance Review and Assessment Service (30-60 days), new medical technology assessment by the National Evidence-based Healthcare Collaborating Agency (250 days), and health insurance registration (100 days). This process could take up to 490 days, leading to criticism from the industry that regulatory procedures could not keep pace with technological advancements.
In response, the Ministry of Health and Welfare and the Ministry of Food and Drug Safety simultaneously revised the "Regulations on the Assessment of New Medical Technologies" and the "Notification on Medical Device Approval, Registration, and Review." As a result, these medical devices can now enter the market in as little as 80 days, or at most within 140 days, after completing the approval and existing technology confirmation procedures.
The beneficiaries of this new system are 199 items announced by the Ministry of Food and Drug Safety. This includes 113 digital medical devices such as AI-based standalone software, 83 in vitro diagnostic medical devices such as diagnostic reagents, and 3 medical robots such as automated system surgical robots.
To prevent the overuse of non-reimbursed services and reduce patients' financial burden when using immediate market entry medical technologies, the government will allow the Minister of Health and Welfare to conduct a new medical technology assessment by authority during the immediate entry period if necessary, and to determine whether the technology will be covered by health insurance. If the assessment results are insufficient, the technology will be withdrawn from the market.
Kwak Soonheon, Director of Healthcare Policy at the Ministry of Health and Welfare, stated, "Unsafe medical technologies will be removed from the market, and we will monitor the use of non-reimbursed services to reduce patient burdens. We will continue to cooperate with relevant agencies to ensure the new system is established in clinical practice."
Lee Namhee, Director General of the Medical Device Safety Bureau at the Ministry of Food and Drug Safety, said, "We expect this to resolve difficulties faced by companies utilizing innovative new technologies in medical devices when entering the market, and to expand access by providing treatment opportunities to patients in need of new medical technologies. We also plan to ensure the safety of medical devices through strengthened clinical evaluation data during approval and certification."
Details of the amended regulations can be found on the National Law Information Center, as well as the websites of the Ministry of Health and Welfare and the Ministry of Food and Drug Safety.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










