Moderna's Cancer Vaccine Receives Accelerated Approval... Commercialization Roadmap Formalized
SillaJen's Oncolytic Virus-Based Universal Cancer Vaccine Platform in the Spotlight
HLB and Aston Science Join Global Competition with Next-Generation Del
As global pharmaceutical giant Moderna formalizes its roadmap for the commercialization of cancer vaccines, the related market is heating up rapidly. With cancer vaccines emerging as a treatment strategy set to be introduced into real-world medical settings-moving beyond the research phase-domestic bio companies with proprietary platform technologies are drawing increased attention.
According to the industry on January 22, Moderna recently announced at the JP Morgan Healthcare Conference (JPMHC) its plan to apply for accelerated approval from the U.S. Food and Drug Administration (FDA) for its cancer vaccine. The company cited results from clinical trials of its personalized cancer vaccine, 'mRNA-4157(V940)', co-developed with Merck (MSD) in the United States, which reduced the risk of recurrence or death by 49% in melanoma patients.
Cancer vaccines are therapies that induce the immune system to attack cancer by making it recognize antigens characteristic of cancer cells. In particular, when combined with immuno-oncology drugs, they can enhance treatment efficacy, making them a next-generation immunotherapy strategy. Moderna’s recent clinical results are seen as a signal that cancer vaccines have moved beyond concept validation and now have real commercial potential.
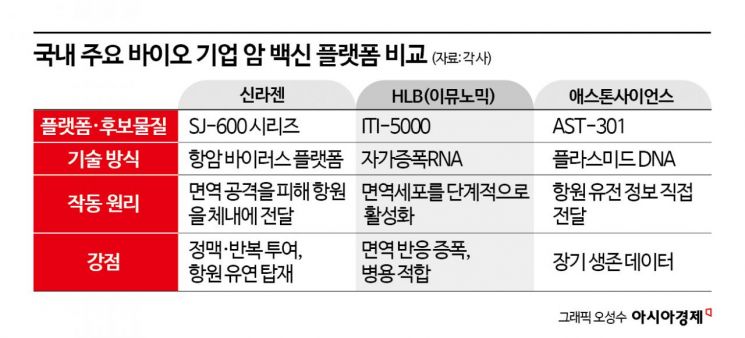
As the commercialization of cancer vaccines becomes more tangible, Korean bio companies with proprietary cancer vaccine technologies are also being spotlighted. SillaJen is seeking to expand into the cancer vaccine field through its next-generation oncolytic virus platform, the 'SJ-600 series.' SJ-600 is a delivery platform that can flexibly load various antigens depending on the therapeutic purpose. The platform expresses the protein 'CD55' on the virus surface to suppress complement responses of the immune system, helping it evade attacks from neutralizing antibodies in the bloodstream. It is characterized by the ability to be administered intravenously and repeatedly. Its minimal restriction on gene size allows for the inclusion of diverse genetic information, such as patient-specific antigens, which is considered a competitive advantage as a cancer vaccine platform. Lee Dongsup, a professor at Seoul National University College of Medicine, previously evaluated at the Korean Society for Cell Biology’s academic conference that it functions as a 'universal platform' capable of flexibly loading and delivering various antigens for different therapeutic purposes.
There are also cases entering the clinical stage. HLB’s U.S. subsidiary, Immunomic Therapeutics, recently received FDA approval for a Phase 1 Investigational New Drug (IND) application for its self-amplifying RNA (saRNA)-based cancer vaccine candidate, 'ITI-5000.' ITI-5000 delivers cancer antigens to the lysosome, an intracellular digestive organelle in immune cells, thereby activating 'CD4+ T cells' that coordinate immune responses. This, in turn, induces a cascade immune response stimulating both CD8+ T cells and B cells. The company plans to conduct combination clinical trials with the immuno-oncology drug Keytruda in patients with triple-negative breast cancer, who are at high risk of recurrence.
Aston Science is conducting global clinical trials of 'AST-301,' a therapeutic cancer vaccine based on plasmid DNA (pDNA) that directly delivers antigen genetic information. With approval from the Taiwan Food and Drug Administration (TFDA), Phase 2 clinical trials are underway in HER2-positive gastric cancer patients. According to the company, Phase 1 trials yielded meaningful results in both overall survival (OS) and progression-free survival (PFS). HER2-positive gastric cancer is known to lack standard therapies that prevent recurrence after surgery.
Some experts suggest that the key to the race for cancer vaccine commercialization lies not in the antigens themselves, but in platform technologies that ensure efficient delivery and repeated administration. An industry insider stated, "As the commercialization of cancer vaccines comes into view, companies with safe and efficient antigen delivery technologies will attract greater attention," adding, "The technological value of domestic companies with diverse modality-based platforms may also be re-evaluated."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















