Remegen and SciNeuro Pharmaceuticals of China
Sign Trillion-Won Technology Transfer Deals with Novartis and AbbVie
Combination Therapies and Drug Delivery Technologies...
"Maximizing ADC Efficacy"
The news of trillion-won contracts signed by Chinese biotech companies at this year's JP Morgan Healthcare Conference (JPMHC) is being seen as evidence of big pharma's new strategic approach to the antibody-drug conjugate (ADC) market.
Industry analysts note that the sector has moved beyond the simple stage of pipeline expansion and entered a phase of competing for improved outcomes. Rather than betting on a single antibody or drug, attention is now focused on "enabling technologies" that can enhance the overall effectiveness of existing ADCs.
According to the biotech industry on January 19, during the JPMHC held in San Francisco, USA, starting January 12 (local time), Chinese biotech companies drew significant attention by announcing a series of "mega-deals" in the bio market.
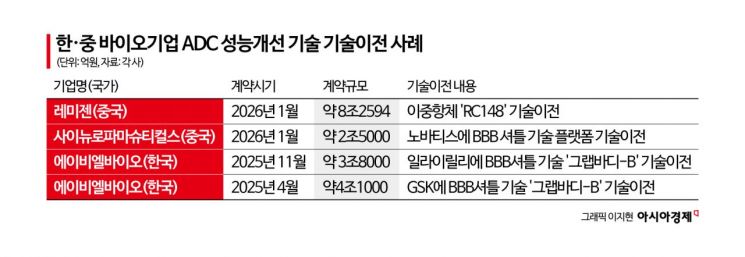
AbbVie signed a technology transfer agreement worth a total of $5.6 billion (about 8.26 trillion won), including an upfront payment of $650 million (about 957.4 billion won), for the anti-cancer drug candidate "RC148" developed by China's Remegen. Novartis announced a technology transfer agreement with Chinese biotech company SciNeuro Pharmaceuticals for a blood-brain barrier (BBB) shuttle platform, valued at $1.7 billion (about 2.5 trillion won), with an upfront payment of $165 million (about 243.3 billion won).
Industry experts point out that both deals share the commonality of investing not in ADCs themselves, but in technologies that help overcome the limitations of existing ADC therapies and expand their applications.
RC148 is a bispecific antibody that simultaneously targets PD-1, an immune checkpoint in tumors, and vascular endothelial growth factor (VEGF). By improving the tumor microenvironment, it is attracting attention as a combination therapy partner that can enhance the efficacy of anti-cancer drugs, including ADCs.
SciNeuro Pharmaceuticals’ BBB shuttle platform helps antibodies or ADCs cross the blood-brain barrier. It is regarded as a technology that can expand the application of antibody-based therapies to central nervous system diseases, where traditional treatments have struggled to reach.
The focus of big pharma on complementary technologies rather than ADCs themselves is interpreted as a reflection of the ADC market's transition from mere pipeline expansion to a stage where efficacy and application scope are the main areas of competition. Rather than betting on a single antibody or new drug, "enabling technologies" that can boost the performance of existing ADCs are emerging as new investment keywords.
Global consulting firm Bain & Company stated in its recently released "2026 Pharmaceutical M&A Report" that "leading companies in the industry, with pipelines full of ADCs, are now focusing on acquiring delivery technologies and combination mechanisms that can maximize drug efficacy."
In fact, Novartis, which has not traditionally been classified as a major player in ADCs, is now strengthening its strategy to expand the scope of antibody-based therapies. Last year, it acquired US biotech company Avidity Biosciences to secure RNA delivery technology using antibodies, and with the adoption of the BBB shuttle technology, the company is investing in platforms that overcome delivery limitations rather than in ADCs themselves.
AbbVie is a representative big pharma company that has already built a robust ADC pipeline. In 2023, it acquired ImmunoGen, a company specializing in ADCs, to secure ADCs for ovarian cancer treatment. Since then, AbbVie has focused on expanding additional indications and combination therapies. The introduction of RC148 is also interpreted as an effort to secure a combination therapy partner that can enhance the effectiveness of existing anti-cancer treatments, rather than simply adding a new ADC.
Industry observers believe that as the center of gravity in ADC competition shifts from individual drug candidates to delivery and microenvironment improvement technologies, interest in platform technologies developed in countries such as Korea and China will continue for the time being.
Last year, Korean biotech company ABL Bio licensed its BBB shuttle bispecific antibody platform "Grabody-B" to GSK in a deal worth approximately 4.1 trillion won. In November of the same year, the company successfully signed an additional technology transfer agreement with Eli Lilly worth about 3.8 trillion won, achieving a series of large-scale, trillion-won contracts.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















