Held in San Francisco from January 12 to 15
Major Korean Bio Companies Including Samsung Biologics and Celltrion to Participate
The world's largest bio investment event, the JP Morgan Healthcare Conference (JPMHC), is just a week away. With the total value of technology transfers exceeding 21 trillion won last year, Korean pharmaceutical and biotech companies, which have made their presence felt in the global bio industry, are set to participate in large numbers, actively engaging in new discussions on collaboration and investment.
According to industry sources on January 5, the JPMHC will be held from January 12 to 15 (local time) at the Westin St. Francis Hotel in San Francisco, United States. It is a gathering where global big pharma companies and investors come together to share mid-to-long-term strategies and discuss partnerships. Every year, it has served as a signal for major 'big deals' in technology transfer and joint development. Global pharmaceutical giants such as Pfizer, Johnson & Johnson, and Eli Lilly will also announce their R&D status and mid-to-long-term plans on the official JPMHC stage.
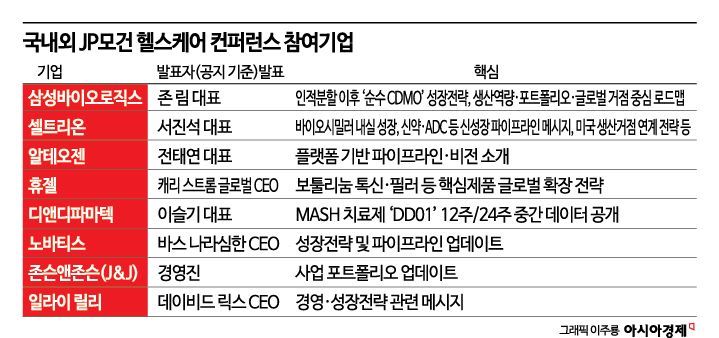
At this event, five companies-Samsung Biologics, Celltrion, Alteogen, D&D Pharmatech, and Hugel-have been officially invited by the organizers to take the stage for presentations. Industry insiders expect Samsung Biologics and Celltrion to deliver messages on the main track, while Alteogen, D&D Pharmatech, and Hugel will present on the Asia-Pacific (APAC) track. Other companies, including Yuhan Corporation, Hanmi Pharmaceutical, Samsung Bioepis, SK Biopharmaceuticals, Lotte Biologics, and Onconic Therapeutics, will seek global partners through unofficial meetings rather than official presentations.
Samsung Biologics has received an official invitation for ten consecutive years since 2017. CEO John Rim will give a presentation on the second day of the event alongside GSK, AstraZeneca, and Eli Lilly. The topic will be the new CMO (contract manufacturing organization) brand 'ExellenS,' with the company's core value '4E (Excellence)' at the forefront. He will introduce last year's achievements, this year's business plans, long-term vision, and competitiveness. During the event, the company will focus on meetings with investors and potential clients, promoting its global CDMO competitiveness and networking to secure additional orders and expand its business.
Celltrion is expected to have CEO Seo Jinseok attend and directly explain the company's business strategy. As the company accelerates its transformation from a biosimilar-focused company to a new drug developer, it is likely to expand its partnerships by disclosing the current status of its ADC (antibody-drug conjugate) new drug development. Lotte Biologics will have CEO Shin Yooyeol, CEO James Park, and other executives present to seek opportunities for orders and partnerships. With three orders secured this year focused on candidate substances and clinical stages, the company aims to achieve a 'large-scale contract' that leads to commercialization.
Alteogen, D&D Pharmatech, and Hugel will present on the Asia-Pacific (APAC) track at this year's JP Morgan Healthcare Conference. For Alteogen, newly appointed CEO Jeon Taeyeon, who took office on December 26, 2025, will take the lead, while Hugel's global CEO Carrie Strom, who joined in October 2025, will be at the forefront. Both new CEOs are tasked with leading global issues, making their presentations highly anticipated.
Hugel launched its botulinum toxin product 'Botulax' (marketed as Letibot in the United States) in the U.S. in March last year. In a previous JPMHC presentation, the company set a goal of capturing a 10% share of the U.S. toxin market within three years of launch. D&D Pharmatech, with the possibility of global technology transfer being discussed this year, plans to announce interim results from week 24 of the Phase 2 clinical trial for its MASH (metabolic dysfunction-associated steatohepatitis) treatment candidate 'DD01' at this event, attracting significant attention.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















