On December 23, Lunit, a medical artificial intelligence (AI) company, announced that the results of a study using its AI biomarker platform, 'Lunit Scope,' for diagnosing HER2 (human epidermal growth factor receptor 2) in patients with advanced bile duct cancer have been published in the official journal of the United States and Canadian Academy of Pathology (USCAP), 'Laboratory Investigation (IF 4.2)'.
Bile duct cancer is a rare cancer with a poor prognosis. Recently, clinical studies on HER2-targeted therapies such as Enhertu (ingredient: trastuzumab deruxtecan) and Zherha (zanidatamab) have become more active, increasing the importance of HER2 diagnostics for selecting patients eligible for these treatments. However, HER2 immunohistochemistry (IHC) test results can vary depending on the pathologist's interpretation, leading to ongoing calls for a more consistent evaluation system.
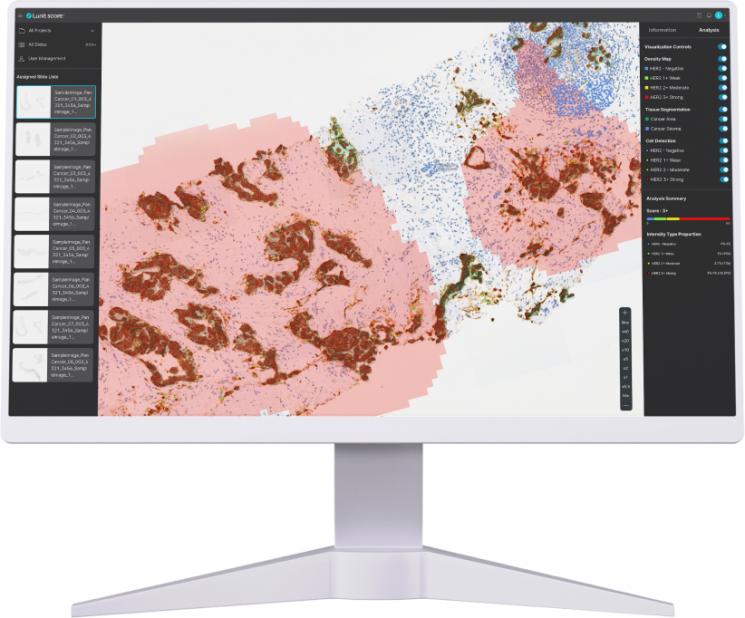 Lunit's AI biomarker platform, 'Lunit Scope,' is reviewing HER2 IHC stained slides of 291 bile duct cancer patients. Lunit
Lunit's AI biomarker platform, 'Lunit Scope,' is reviewing HER2 IHC stained slides of 291 bile duct cancer patients. Lunit
This study was jointly conducted by researchers from Lunit, Bundang CHA Hospital, and Ilsan CHA Hospital. It quantified the differences in interpretation among pathologists in the HER2 IHC evaluation of patients with advanced bile duct cancer and analyzed the degree to which the AI model's results matched the consensus of the pathologists.
The research team analyzed 309 HER2 IHC stained slides from 291 patients with advanced bile duct cancer who received systemic anti-cancer treatment at Bundang CHA Hospital between 2019 and 2022. Three pathologists independently interpreted the slides using both optical microscopy and digital pathology methods, and these results were compared with those from Lunit Scope HER2.
The analysis showed that the rate at which all three pathologists provided the same interpretation was 62.1% for optical microscopy and 63.4% for digital pathology, confirming the existence of inter-observer variability. In contrast, Lunit Scope showed an 83.5% concordance rate with the consensus of the pathologists, demonstrating relatively superior agreement. Additionally, the concordance between the AI and pathologists was higher with digital pathology than with optical microscopy.
This study is significant in that it quantitatively demonstrated that an AI-based digital pathology system can improve the reproducibility and consistency of HER2 evaluation in patients with the rare cancer, bile duct cancer. This is expected to help more precisely select patients eligible for HER2-targeted therapies in the future.
Based on this study, Lunit plans to expand research into more detailed areas such as low HER2 expression, and to broaden the application of digital pathology-based AI solutions through additional multicenter collaborative studies.
Seo Bumseok, CEO of Lunit, said, "Due to the nature of HER2 diagnostics, discrepancies among pathologists are inevitable. The significance of this study lies in demonstrating that AI can reduce such discrepancies and enhance objectivity and reproducibility. Lunit will continue to lead the standardization of HER2 diagnostics across various cancer types, including bile duct cancer, thereby contributing to expanding treatment opportunities for patients."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















