For Adults Aged 60 and Older
and High-Risk Adults Aged 18 to Under 60
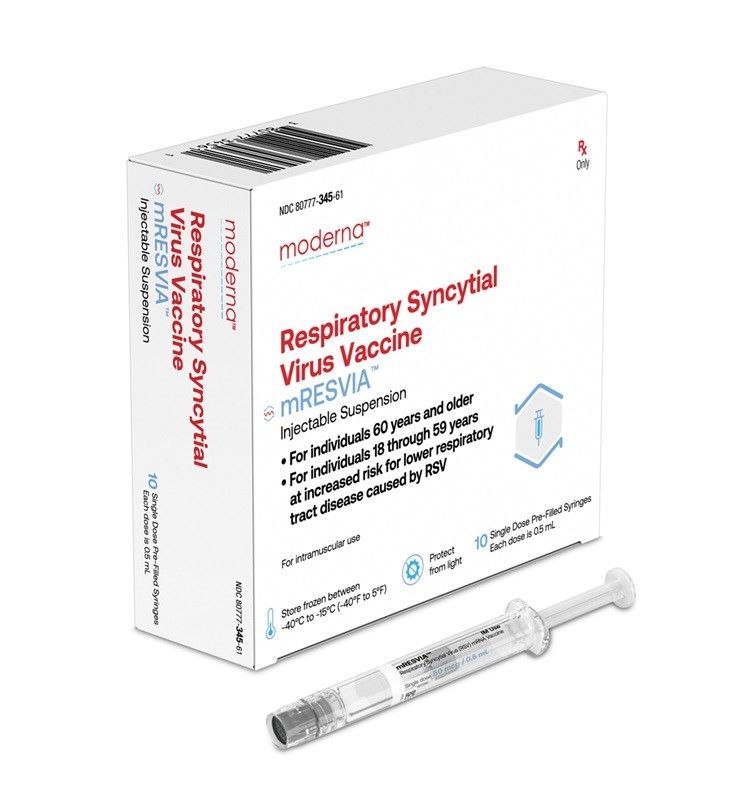
Moderna Korea announced on December 19 that its respiratory syncytial virus (RSV) mRNA (messenger ribonucleic acid) vaccine, "Mres B Pre-Filled Syringe," has been approved by the Ministry of Food and Drug Safety. This is the first mRNA platform vaccine approved in Korea for RSV prevention.
Mres B Pre-Filled Syringe has been approved for the prevention of lower respiratory tract disease (LRTD) caused by RSV in adults aged 60 and older, as well as in high-risk adults aged 18 to under 60. RSV is a virus that can cause symptoms similar to the common cold and can progress to severe lower respiratory tract infections such as pneumonia. The disease burden is known to be particularly high among the elderly and adults with underlying health conditions.
Kim Sangpyo, CEO of Moderna Korea, stated, "RSV is a major respiratory disease that can lead to hospitalization and severe complications in older adults and those with underlying health conditions. Mres B Pre-Filled Syringe is the second Moderna product approved in Korea, and following COVID-19, we are very pleased that Moderna’s mRNA technology can now also contribute to public health by preventing RSV."
The approval of Mres B Pre-Filled Syringe was based on positive results from the phase 3 "ConquerRSV" clinical trial, which was a randomized, placebo-controlled, observer-blinded, event-driven study conducted in 22 countries, including Korea, with approximately 37,000 adults aged 60 and older. No significant safety concerns were identified in this phase 3 trial.
With this domestic approval, Moderna expects to demonstrate the potential for expanding mRNA technology in the field of RSV prevention and to contribute to the prevention of RSV among a broader age group, including high-risk adults.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















