Professor Kim Gwiyong's Team Develops Technology to Extract Over 95% of Nickel and Cobalt from Waste Batteries
Special Solvent Solves Indiscriminate Extraction Issue; Published in Energy Storage Materials
A domestic research team has developed a recycling technology that recovers nickel and cobalt from waste batteries with ultra-high purity of 99% and a recovery rate of over 95%.
This is an eco-friendly and highly efficient technology that overcomes the limitations of conventional hydrometallurgical recycling methods, which involve complex chemical processes and generate large amounts of wastewater. It is expected to become a "game changer" that could transform the battery recycling industry.
The team led by Professor Kim Gwiyong from the Department of Urban and Environmental Engineering at UNIST has succeeded in selectively separating and recovering nickel and cobalt from waste batteries using an electrochemical process that employs multifunctional special solvents.
 Research team, (from left) Professor Kim Gwiyong, Researcher Choi Seongmin (first author). Provided by UNIST
Research team, (from left) Professor Kim Gwiyong, Researcher Choi Seongmin (first author). Provided by UNIST
Waste batteries are often referred to as "urban mines" because they contain large quantities of strategic metals such as nickel, cobalt, and manganese. However, since these metals are mixed together, it is difficult to separate and reuse them. The conventional process requires the use of strong acids like sulfuric acid and chemical extractants, which not only generate hazardous wastewater but also involve multi-step processes with low energy efficiency.
The electrochemical process developed by the research team minimizes the use of chemicals and the generation of wastewater, while also improving both purity and recovery rate in a single process.
The method involves dissolving waste battery powder into a liquid, then applying varying voltages to precipitate the metal ions into solid metals. The technology utilizes the principle that each metal ion precipitates into a solid at a different voltage.
Nickel and cobalt, which account for about 50% of battery manufacturing costs, have the issue of precipitating together at similar voltages. This problem was solved by using special solvents (deep eutectic solvents). The ethylene glycol component of the special solvent binds with nickel ions, while the chloride component binds with cobalt ions, thereby changing the voltages at which the two metal ions are precipitated as solids. As a result, nickel is separated and extracted at a voltage of -0.45V, while cobalt is separated and extracted at a voltage of -0.9V.
Additionally, the chlorine component that naturally occurs during the process selectively dissolves only the cobalt that is mixed as an impurity, thereby increasing the separation purity of nickel without the need for an additional purification process. The chlorine used to dissolve cobalt is ionized into hydrochloric acid ions, so there is no concern about atmospheric emissions. The accumulated hydrochloric acid component in the solvent can be regenerated into pure hydrochloric acid for reuse.
When this technology was applied to commercial NCM (nickel, cobalt, manganese) waste batteries, both nickel and cobalt were separated with a purity of over 99.9%, and both metals achieved a recovery rate of over 95%.
The special solvent used maintained its performance even after being reused more than four times, further minimizing wastewater generation.
Professor Kim Gwiyong stated, "We have simultaneously solved the trade-off between purity and recovery rate, which has been a chronic limitation of electrochemical separation methods. By minimizing the use of chemicals and the generation of wastewater while ensuring economic feasibility, this technology will contribute to establishing a sustainable battery circular economy."
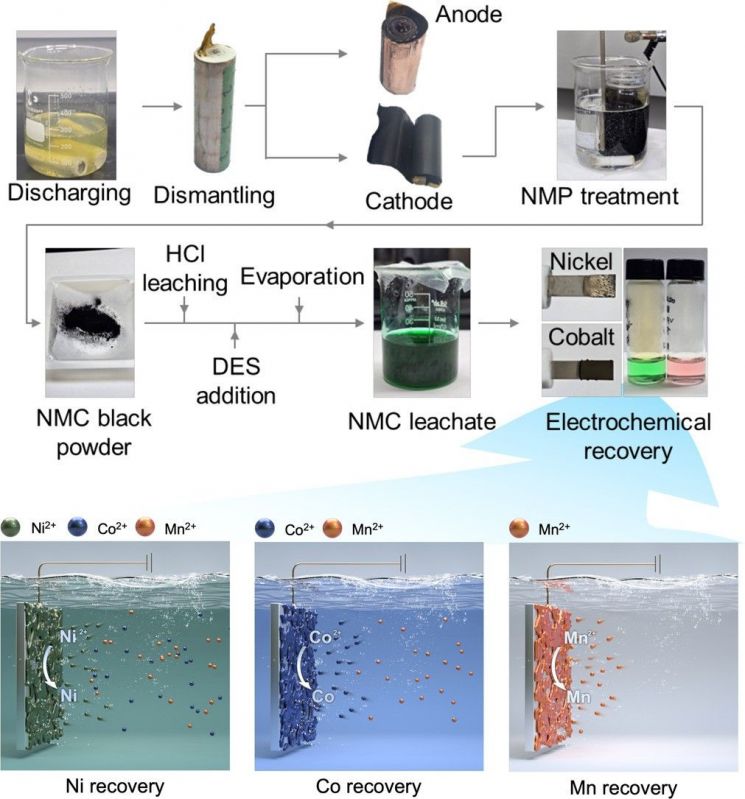 Schematic diagram of the sequential metal recovery process from waste batteries based on electrochemical deep eutectic solvents (special solvents).
Schematic diagram of the sequential metal recovery process from waste batteries based on electrochemical deep eutectic solvents (special solvents).
The results of this research were published in the October issue of the international journal Energy Storage Materials.
The research was supported by the Ministry of Education, the National Research Foundation of Korea, and UNIST.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










