Ministry of Food and Drug Safety and Pharmaceutical Companies
Hold Roundtable on "Essential Medicines Public Production and Distribution Network"
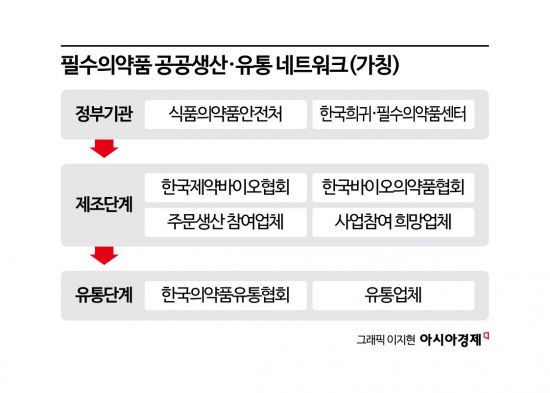
The Ministry of Food and Drug Safety announced on September 26 that it has established the "Essential Medicines Public Production and Distribution Network" in cooperation with the Korea Orphan & Essential Drug Center, in order to actively implement the national policy task of "Stabilizing and Supporting the Supply of National Essential Medicines."
The Essential Medicines Public Production and Distribution Network is designed to comprehensively support the supply chain, from the initial stage of manufacturing essential medicines to distribution. Its goal is to systematically discuss the selection of eligible items and administrative and technical support measures to promote the future made-to-order production system, and to incorporate necessary incentives for pharmaceutical companies into the system.
During a roundtable held on the same day, attended by government officials, pharmaceutical industry associations, and companies interested in made-to-order production and distribution of national essential medicines, the Ministry of Food and Drug Safety and the Korea Orphan & Essential Drug Center shared the current status of the made-to-order production project for essential medicines and outlined future directions for operating the network.
The national essential medicines made-to-order production project is a government-supported initiative that utilizes the production capabilities of private pharmaceutical companies to ensure a stable domestic supply of items whose supply has been discontinued or is at risk of being discontinued. Currently, six pharmaceutical companies are participating in the project, producing seven national essential medicines. Industry representatives shared the difficulties they have experienced while participating in the made-to-order production project and suggested improvements to the system and support measures needed to further promote the project in the future.
The Ministry of Food and Drug Safety stated, "Going forward, we will do our utmost to strengthen the domestic production system for national essential medicines through public-private cooperation centered on the Essential Medicines Public Production and Distribution Network, so that medicines can be supplied stably to medical sites and patients."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.