European Association for the Study of Diabetes Opens on September 15
Amgen, Metsera Develop Once-Monthly Injectable Formulations
Novo Nordisk and Eli Lilly Pursue 'Next GLP-1' with Multi-Action Drugs Targeting GIP and Amylin
The competition to develop obesity treatments that are more convenient and powerful than Wegovy and Mounjaro is intensifying. Since the popularization of GLP-1 (glucagon-like peptide-1) class therapies, pharmaceutical companies have been expanding their efforts to achieve stronger weight loss effects, reduce dosing burden, and broaden indications to include cardiovascular diseases.
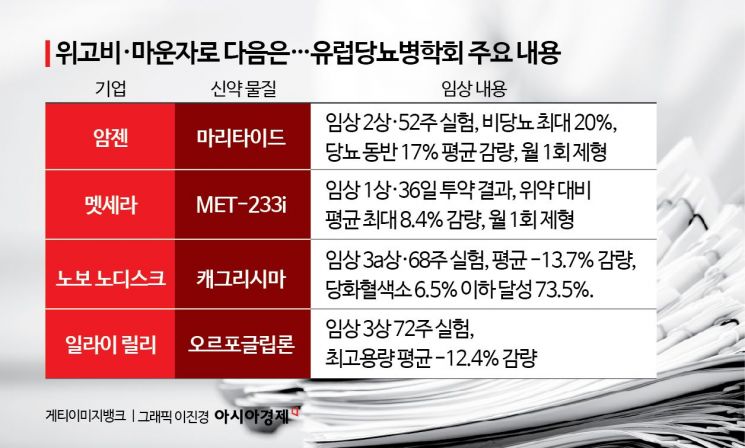
On September 15 (local time), at the European Association for the Study of Diabetes (EASD 2025) held in Vienna, Austria, the most notable trend was the competition to "reduce injection intervals." A prime example is Amgen's once-monthly formulation, "Maritide." This new drug combines GLP-1 activity with antagonism (blocking) of the GIP (gastric inhibitory polypeptide) receptor. GIP is secreted from the gut after food intake and promotes insulin secretion. Excessive GIP secretion leads to fat accumulation, and this function of blocking GIP secretion has been applied to obesity treatments. This is the same mechanism as Mounjaro. In a phase 2 trial over 52 weeks, it showed an average weight loss of up to about 20% in non-diabetic patients and up to about 17% in patients with type 2 diabetes. By suggesting the possibility of once-monthly or even longer dosing intervals, it is challenging the "once-weekly" standard.
US biotech company Metsera introduced its ultra-long-acting amylin analog "MET-233i" at this conference. Amylin is a hormone secreted from the pancreas along with insulin, released after meals to prevent sudden spikes in blood sugar and to maintain satiety for longer. This candidate is administered once a month, and in early clinical trials, it showed an average weight loss of up to 8.4% compared to placebo at 36 days after administration, raising the possibility of commercializing a once-monthly combination therapy of amylin analog and GLP-1. Early safety signals also appeared favorable.
There are also active efforts to raise the ceiling on efficacy. Novo Nordisk is developing "Cagrisima," which combines semaglutide (the active ingredient in Wegovy) with the amylin analog cagrilintide, as a new treatment to follow Wegovy. In a phase 3 trial over 68 weeks, this new drug achieved an average weight loss of -13.7% and a 73.5% rate of achieving HbA1c (glycated hemoglobin) below 6.5%. Eli Lilly has unveiled "Retatrutide," a triple agonist targeting GLP-1, GIP, and glucagon, demonstrating the trend toward multi-target diabetes and obesity treatments.
Hanmi Pharmaceutical is also presenting preclinical research results on new-concept obesity treatments such as "HM17321" at this conference. HM17321 is being developed as an obesity treatment that simultaneously achieves weight loss and muscle gain. At this conference, for the first time globally, the molecular biological mechanism for muscle gain by HM17321 will be elucidated through proteomic research on laboratory mice.
The goals of treatment go beyond simple weight loss. Semaglutide has expanded the market with data showing reduced risk of cardiovascular death and myocardial infarction, and multi-action drugs such as Cagrisima are evolving by simultaneously demonstrating improvements in cardiovascular metabolic indicators such as HbA1c, blood lipids, and blood pressure. The industry expects that, going forward, diabetes and obesity treatments will actively expand indications to include non-alcoholic fatty liver disease and heart failure.
Industry experts believe that as the era of high-dose and multi-action drugs arrives, the competition for "hard outcomes"-actual clinical results-covering not only weight loss but also cardiovascular and hepatic metabolic comorbidities will intensify. Companies that can prove both dosing convenience and systemic benefits are likely to seize next-generation leadership. Kim Seona, a researcher at Hana Securities, said, "In the future, obesity treatments will focus on long-acting formulations for convenience and on combination therapies for greater weight loss. This approach can address the issue of dosage escalation limits due to side effects."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.