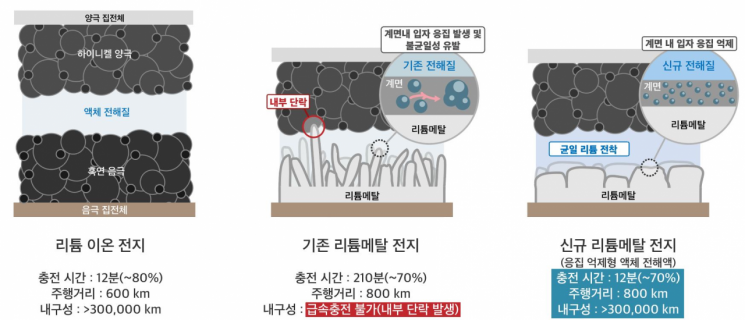
"If lithium metal batteries are applied to electric vehicles, battery charging time can be shortened to 12 minutes, and the driving range can be increased from a maximum of 600 km to 800 km. The battery lifespan is also expected to exceed a cumulative total of 300,000 km." This achievement was made possible by a domestic research team that resolved the longstanding challenge preventing the application of lithium metal batteries in electric vehicles.
Lithium metal batteries use lithium metal as the anode material instead of graphite, thereby increasing energy density. However, the formation of tree-branch-shaped lithium crystals (hereinafter referred to as dendrites) on the anode surface during charging makes it difficult to ensure battery performance and safety.
The domestic research team has solved this problem, making it possible to apply lithium metal batteries to electric vehicles. This is expected to lay the foundation for a new era in electric vehicle battery technology by dramatically improving charging, driving range, and lifespan.
 (Front row from the left) Kwon Hyukjin, PhD candidate in Bio and Chemical Engineering, Kim Heetak, Professor, and Kim Seongsu, Professor in Mechanical Engineering. Provided by KAIST
(Front row from the left) Kwon Hyukjin, PhD candidate in Bio and Chemical Engineering, Kim Heetak, Professor, and Kim Seongsu, Professor in Mechanical Engineering. Provided by KAIST
KAIST announced on September 4 that a research team from the Frontier Research Laboratory (FRL), led by Professor Kim Heetak of the Department of Bio and Chemical Engineering, has developed a groundbreaking "aggregation-suppressing new liquid electrolyte" core technology that can significantly improve the performance of lithium metal batteries.
The Frontier Research Laboratory was established in 2021 by KAIST and LG Energy Solution to develop next-generation lithium metal battery technology. Professor Kim serves as the director of the laboratory.
Lithium metal batteries are produced by replacing the graphite anode, which is one of the key materials in lithium-ion batteries, with lithium metal.
However, until now, it has been difficult to actually use lithium metal due to the dendrite problem. Dendrites refer to the needle-like lithium deposits (crystals) formed due to the accumulation of lithium ions during the electrodeposition process inside the battery.
The dendrite phenomenon becomes more pronounced during fast charging, causing internal short-circuits in the battery and making it difficult to realize lithium metal batteries that can be recharged under rapid charging conditions.
The FRL joint research team sought to identify the fundamental cause of dendrite formation during the rapid charging process of lithium metal and confirmed that non-uniform interfacial aggregation reactions on the lithium metal surface contribute to dendrite formation. They also succeeded in developing an "aggregation-suppressing new liquid electrolyte" that can address this issue.
The new liquid electrolyte utilizes an anion structure with weak binding force to lithium ions, minimizing non-uniformity at the lithium interface and effectively suppressing dendrite growth during rapid charging.
This technology maintains high energy density while overcoming the slow charging speed, which has been a major limitation of conventional lithium metal batteries. Above all, it enables stable operation even during long-distance driving and rapid charging.
Kim Jaeyoung, CTO and Executive Vice President of LG Energy Solution, said, "LG Energy Solution and KAIST have been collaborating through the FRL for the past four years, and we are generating meaningful results. Both sides will continue to focus on solving technological challenges through industry-academia cooperation and achieving the best results in the next-generation battery field."
Kim Heetak, Professor of Bio and Chemical Engineering at KAIST, said, "This research is significant in that it has established a key foundation for solving the technological challenges of lithium metal batteries based on an understanding of interfacial structures. Through this research, we have overcome the biggest obstacle to introducing lithium metal batteries into electric vehicles."
Meanwhile, Kwon Hyukjin, PhD candidate in the Department of Bio and Chemical Engineering at KAIST, participated as the first author in this study. The research results were published the previous day (September 3) in Nature Energy.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.