Safety and Tolerability Demonstrated in US Phase 1 Trial
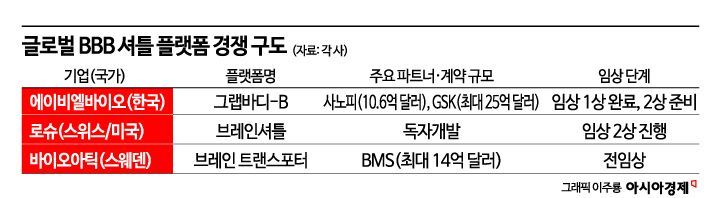
Roche, BMS, and Others Advancing New Drugs Based on BBB Platform
The new Parkinson's drug developed by the Korean biotech company ABL Bio has shown positive results in a Phase 1 clinical trial in the United States, increasing the likelihood of entering the neurodegenerative disease market. ABL Bio's progress, which leverages its blood-brain barrier (BBB) shuttle platform technology, is drawing attention in a market where global pharmaceutical giants are actively competing.
According to the industry on September 2, ABL Bio's bispecific antibody candidate for treating neurodegenerative brain diseases, 'ABL301,' has demonstrated safety and tolerability in a Phase 1 clinical trial in the United States. The trial, conducted from December 2022 to April this year with 91 adult participants, confirmed safety and tolerability in both the single ascending dose (SAD) and multiple ascending dose (MAD) studies. No deaths or serious adverse events occurred in any of the participants.
ABL Bio CEO Lee Sanghoon stated, "The results of this Phase 1 trial will serve as important evidence for Sanofi to proceed with subsequent clinical studies," and added, "Neurodegenerative brain diseases, including Parkinson's disease, pose a significant threat to the lives of patients and their families, but there is a large unmet need due to the lack of fundamental treatments." According to market research firm Zion Market Research, the size of the neurodegenerative disease market was approximately $61.93 billion (about 86 trillion won) last year, and it is expected to grow to around $121.3 billion (about 169 trillion won) by 2034. The compound annual growth rate (CAGR) over the next ten years is projected to reach about 7.1%.
Neurodegenerative diseases such as Parkinson's and Alzheimer's refer to conditions in which nerve cells are gradually damaged and die, resulting in diminished function of the brain and spinal cord. The biggest challenge in developing related therapeutics is the blood-brain barrier (BBB). The BBB serves as a protective shield that blocks external toxins from entering the brain, but at the same time, it also prevents therapeutic drugs from reaching the brain. The BBB shuttle platform is a technology that enables the transport of antibodies or peptides by binding them to protein receptors capable of crossing this barrier. Competition between major pharmaceutical companies and biotech ventures to develop such platforms is intensifying.
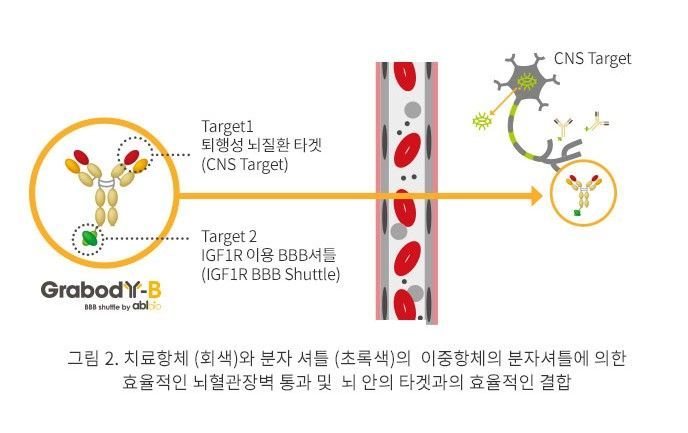
In Korea, ABL Bio is leading the way. The company has developed the 'GrabBody-B' platform, which utilizes the 'IGF-1 receptor,' a protein receptor that regulates cell growth, metabolism, and neural function. ABL Bio signed a technology transfer agreement with Sanofi of France worth $1.06 billion (about 1.4771 trillion won), and recently secured a major deal with GSK of the United Kingdom worth up to 4.1 trillion won. This platform can be expanded beyond antibodies to various new modalities, raising expectations for applications not only in Parkinson's disease treatment but also across a wide range of neurological disorders.
Global competition in the BBB shuttle platform field is also fierce. Roche of Switzerland is developing 'Trontinemab,' an Alzheimer's antibody candidate, using its 'Brain Shuttle' platform that leverages the transferrin receptor (TfR1). The company has reported results showing more than a 50-fold increase in brain penetration compared to existing approaches and is currently conducting Phase 2 clinical trials. Bristol Myers Squibb (BMS) has signed a deal worth up to $1.4 billion to license the BBB shuttle platform 'Brain Transporter' from Swedish company BioArctic, which utilizes an iron-ion transport mechanism.
An industry official commented, "The essence of competition in the BBB shuttle platform space is who can cross the BBB more safely and efficiently," and added, "ABL Bio's success will enhance trust in the original technologies held by Korean biotech companies on the global stage and strengthen incentives for technology development and investment among latecomers."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.