Reducing Power Consumption for Hydrogen Production
and Immediate Storage in Liquid Organic Compounds
Using the Biocofactor FAD:
Professor Song Hyunkon’s UNIST Team’s Breakthrough
A new technology has been developed that can reduce the electricity required for hydrogen production, making green hydrogen more affordable to produce.
This technology utilizes a biocofactor involved in cellular energy metabolism to lower power consumption. The produced hydrogen can be stored directly in a liquid organic compound without passing through a gaseous state, which not only reduces hydrogen production costs but also cuts storage and transportation expenses. As a result, both academia and related industries are paying close attention to this advancement.
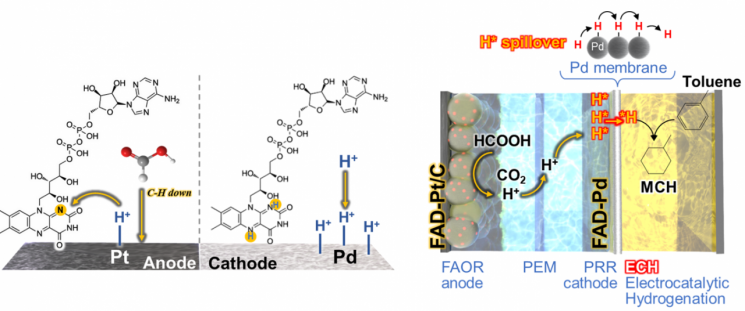
On August 26, UNIST announced that Professor Song Hyunkon's team from the Department of Energy and Chemical Engineering has developed an electrochemical system that coats the electrode surfaces with the biocofactor FAD, enabling low-voltage hydrogen production and immediate storage in a liquid organic compound.
According to the research team, the system consists of platinum (Pt) and palladium (Pd) electrodes. At the platinum electrode, formic acid (HCOOH) is oxidized, and the resulting electrons travel to the palladium electrode, where they combine with hydrogen ions to produce hydrogen. The generated hydrogen then passes directly through the palladium metal membrane and is stored in the liquid organic compound behind it.
The team coated both electrodes with the FAD cofactor to enhance reaction efficiency and reduce the electricity required for hydrogen production. When measuring the system's voltage during actual hydrogen production and storage, they recorded a low cell voltage of approximately 0.6V, representing a reduction of about 65% compared to conventional systems.
The lifespan of the system also increased eightfold compared to previous approaches, showing no performance degradation even after more than 100 hours of continuous operation. Higher cell operating voltages lead to greater power consumption and reduced lifespan.
Another advantage of this technology is that it eliminates the need for a separate process to inject gaseous hydrogen into the liquid organic compound.
Lee Jisoo, the first author of the study, explained, "When storing hydrogen in a liquid organic compound, conventional methods require injecting gaseous hydrogen (H₂) at high pressure or additional processes to adjust reaction conditions. However, this technology allows hydrogen generated at the electrode to be stored directly in atomic form (H) in the liquid organic compound."
The FAD (flavin adenine dinucleotide) coated on the electrodes is originally a cofactor that assists in the production of ATP, an energy molecule, in cellular mitochondria. It is capable of transferring both electrons and protons (H+, hydrogen ions). In the developed system, this cofactor plays a customized role at both electrodes as needed.
On the palladium electrode side, the cofactor helps hydrogen ions adhere more effectively to the electrode surface. On the platinum electrode, it assists in removing hydrogen intermediates from the electrode surface. If hydrogen intermediates remain on the surface, formic acid cannot approach, delaying the reaction. While hydrogen is generated at the palladium electrode, activating the reaction at the platinum electrode is also essential to reduce the overall system's power consumption.
 Professor Song Hyunkon (from the left), Researcher Lee Jisoo (first author), Research Professor Lee Hosik (co-first author).
Professor Song Hyunkon (from the left), Researcher Lee Jisoo (first author), Research Professor Lee Hosik (co-first author).
Professor Song Hyunkon stated, "By integrating the electron and proton transfer properties of biomolecules into the electrochemical system, we have enabled simultaneous hydrogen production and storage. This research presents a new method for storing hydrogen without high-pressure containers, laying the foundation for safe and efficient hydrogen utilization technologies."
The results of this study were published online on July 2 in 'Applied Catalysis B: Environmental and Energy.' The research was supported by the InnoCore program of UNIST hydro*studio, as well as the Ministry of Trade, Industry and Energy, the Korea Institute for the Advancement of Technology, and the National Research Foundation of Korea (NRF).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.