The process by which the core pathological proteins of Alzheimer's disease, "tau" and "amyloid beta," directly communicate and regulate toxicity at the molecular level has been elucidated. This discovery is expected to provide important clues for the identification of biomarkers for early diagnosis and the development of therapeutics for neurodegenerative brain diseases.
KAIST announced on August 24 that the research team led by Professor Im Mihee from the Department of Chemistry, in collaboration with Dr. Lee Youngho's team at the Korea Basic Science Institute (KBSI), has identified at the molecular level that the microtubule (intracellular transport route) binding region of tau directly interacts with amyloid beta, altering the aggregation pathway and simultaneously alleviating cellular toxicity.
Alzheimer's disease is a neurodegenerative brain disorder. It is estimated that more than seven out of ten of the world's 50 million dementia patients suffer from Alzheimer's disease. The disease is characterized by the accumulation of "neurofibrillary tangles" formed by tau aggregation inside cells and "amyloid beta aggregates" formed by clumps of amyloid beta fragments outside cells.
Tau is a protein that, pathologically, plays a role as a transport route for nutrients and signaling molecules inside nerve cells. Amyloid beta fragments are abnormally cleaved forms of amyloid precursor protein, which is involved in brain development, intercellular signaling, and neuronal recovery, after being affected by enzymes in the neuronal cell membrane.
Although the possibility that tau and amyloid beta coexist and interact inside and outside cells has been suggested, a clear molecular-level understanding of how the direct interaction between these two proteins affects the onset and progression of the disease had not been established.
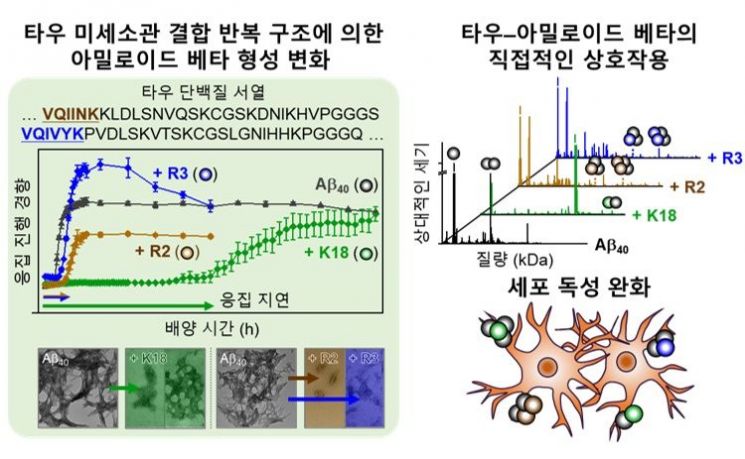
This joint research team is the first in the world to elucidate the molecular-level interaction between these proteins.
According to the joint research team, the tau protein contains structures within nerve cells that bind to microtubules (K18, R1-R4, PHF6, PHF6). Among these, K18, R2, and R3 bind to amyloid beta, forming a "tau-amyloid beta complex (hetero-complex)."
In this process, unlike its original property of forming highly toxic, rigid fibers (amyloid fibrils), amyloid beta binds to specific regions of tau, resulting in reduced toxicity and a shift toward forming less rigid aggregate structures.
 Pathological Changes of Amyloid Beta by Interaction with Tau Microtubule Binding Region. Provided by KAIST
Pathological Changes of Amyloid Beta by Interaction with Tau Microtubule Binding Region. Provided by KAIST
In particular, the repeat structures of tau delayed amyloid aggregation (nucleation stage) associated with disease "onset" and simultaneously altered the aggregation rate and structural characteristics of amyloid beta, which are related to disease "progression." As a result of these processes, the toxicity level caused by amyloid beta was significantly reduced in both the intracellular and extracellular environments of brain cells.
It has been demonstrated that the properties of tau act as a key factor in determining its binding affinity with amyloid beta, aggregation pathway, and toxicity regulation ability.
Professor Im Mihee stated, "This study is significant in that it has identified a new molecular motif that can be used as a therapeutic target not only for Alzheimer's disease but also for various neurodegenerative brain diseases based on protein aggregation."
Dr. Lee Youngho said, "Multidisciplinary research on intermolecular interactions and protein aggregation is expected to play a pivotal role in elucidating the correlations not only between Alzheimer's disease and Parkinson's disease, but also among various diseases such as dementia, diabetes, and cancer."
Meanwhile, this research was conducted with Dr. Kim Mingeun from the Department of Chemistry at KAIST as the first author. The results were published on August 22 in the international journal "Nature Chemical Biology."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

















