Reducing Performance Degradation by Eliminating Moisture That Causes Agglomeration
Published in ACS Energy Letters
The initial performance degradation of an 'anion exchange membrane water electrolyzer (AEMWE)' device, which extracts hydrogen from water, has been found to be caused by the agglomeration of platinum (Pt) catalyst particles on the cathode side.
When a 'dry cathode operation' method, which suppresses agglomeration, was applied, the rate of performance degradation was reduced to half of the previous level. This has secured the long-term reliability of the device and is expected to accelerate the commercialization of green hydrogen production technology.
The research team led by Professor Kwon Youngkook of the Department of Energy and Chemical Engineering at UNIST revealed that, contrary to previous expectations, the initial performance degradation of water electrolyzer devices mainly occurs at the cathode. They also found that 'dry cathode operation', in which liquid electrolyte is not directly supplied to the cathode, is effective in preventing this degradation.
 Research team. (From left) Professor Youngguk Kwon, Researcher Taehun Gong (first author). Provided by UNIST
Research team. (From left) Professor Youngguk Kwon, Researcher Taehun Gong (first author). Provided by UNIST
Water electrolysis is a technology that splits water into hydrogen and oxygen using electricity. Among these, anion exchange membrane water electrolyzers offer advantages such as excellent corrosion resistance and the possibility of lightweight design. However, a persistent issue has been the rapid increase in voltage within the first few hours of operation, leading to a sharp drop in production efficiency?a phenomenon known as 'initial degradation'.
As the voltage rises, more energy is required to produce the same amount of hydrogen, which directly leads to a decrease in efficiency.
The research team found that more than 90% of 'initial degradation' originates from the cathode, where hydrogen gas is generated. This is due to the reduced reactivity caused by the agglomeration of platinum catalyst particles. The main cause of platinum catalyst particle agglomeration was identified as moisture at the cathode.
The team confirmed this finding using a self-developed three-electrode analysis method, instead of the conventional two-electrode method. The two-electrode method only measures the total cell voltage, making it difficult to distinguish which electrode is responsible for performance degradation; it had generally been assumed to be a problem with the anode.
When dry cathode operation was applied to the cathode, the cumulative voltage increase over the initial 40 hours dropped from about 163 mV to 96 mV. This nearly twofold difference means that the voltage rose less over the same period, indicating that hydrogen production efficiency was maintained for a longer time.
First author Kong Taehun, a researcher, explained, "While relatively well-established wet operation conditions are applied to the anode, a mixture of wet and dry conditions has been used for the cathode. This study experimentally demonstrated that, under wet operation, moisture traps hydrogen gas and induces agglomeration of platinum particles, leading to initial degradation. This provides a new operational standard."
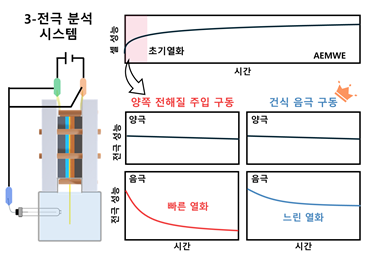 Suppresses initial degradation of the cathode in the hydrogen production device using a dry drive method.
Suppresses initial degradation of the cathode in the hydrogen production device using a dry drive method.
Professor Kwon Youngkook stated, "AEM water electrolysis is a promising candidate for eco-friendly hydrogen production technology, but its commercialization has been limited due to the problem of rapid performance degradation in the early stages of operation. This study provides an important clue for the commercialization of water electrolysis by showing that long-term stability can be improved simply by adjusting operating conditions."
Professor Kwon added, "The new analysis method can also be used for the development of electrode materials, evaluation of cell durability, and optimization of electrode design."
This research was published online on July 3, 2025, in ACS Energy Letters, an internationally renowned journal in the field of energy and environmental science.
The research was supported by the Mid-Career Research Program and the STEAM Research Program of the National Research Foundation of Korea, funded by the Ministry of Science and ICT.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















