Amicogen, a leading company specializing in biopharmaceuticals and bio-materials, announced on July 16 that its partner, Lysando AG, has entered the final stage of commercialization for a spray-type atopic dermatitis product, with the goal of launching in the global market in the fourth quarter of 2025. The product has successfully completed all efficacy and safety evaluations required for commercialization.
In the Human Repeated Insult Patch Test (HRIPT), not a single adverse reaction was reported among all participants with sensitive skin, demonstrating excellent skin compatibility and safety. Additionally, as the world's first spray-type product designed to maintain the natural balance of the skin microbiome, it offers a differentiated approach compared to existing treatments.
Results from double-blind clinical trials showed significant improvement in major symptoms of chronic skin diseases, such as itching and erythema. In a consumer evaluation conducted in the United Kingdom, 83% of participants reported relief from itching, 86% saw improvement in erythema, and over 80% expressed an intention to repurchase, indicating a high level of satisfaction.
The product is currently undergoing certification by the National Eczema Association (NEA) in the United States. Under a distribution strategy led by industry experts with experience at Procter & Gamble, Johnson & Johnson, and Unilever, commercialization targeting a U.S. launch in the fourth quarter of 2025 is anticipated.
Amicogen plans to lead the commercialization of this product in the Korean and Asian markets. The company has maintained a collaborative relationship with Lysando through CDMO (Contract Development and Manufacturing Organization) and strategic partnerships. Amid rising demand for non-steroidal alternative therapies, Amicogen expects this product to establish itself as an innovative solution that combines both safety and efficacy.
An Amicogen official stated, "This product provides a safe solution for alleviating chronic skin diseases without concerns about resistance or long-term side effects," adding, "We expect it to generate a strong market response in Asia, including Korea."
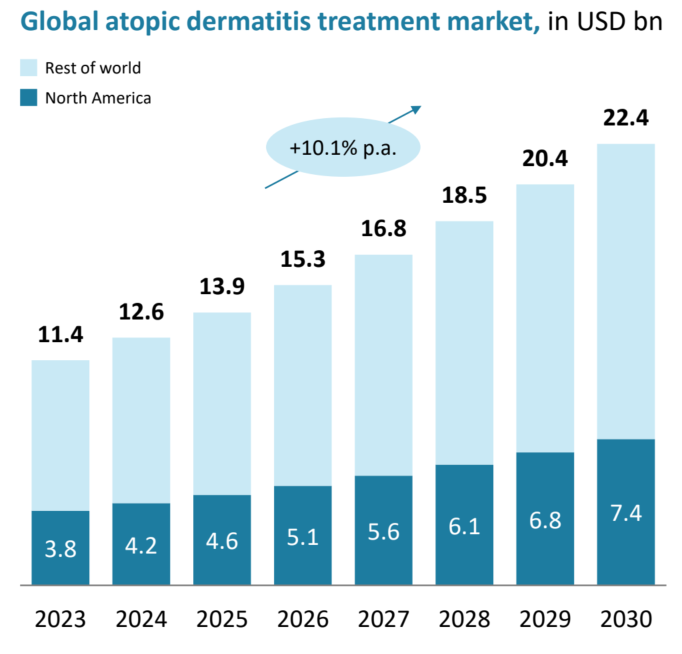
The global atopic dermatitis treatment market is currently valued at approximately $10 billion (about 13 trillion KRW) per year, with a compound annual growth rate (CAGR) of over 10%. The U.S. market alone is projected to reach approximately $1.39 billion (1.8 trillion KRW) in 2025.
Amid this growth, the product is drawing attention as a new solution capable of addressing unmet needs in the market.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.