Professor Jongbeom Baek's Team Achieves 5.6-Fold Increase in Ammonia Yield with Silicon Nitride in Mechanochemical Process
Resource Circulation Achieved by Producing Silicon Nitride from Waste Solar Panels; Published in Nature Communications
The yield of a new ammonia production method, which could replace the current carbon-intensive process, has increased by 5.6 times.
This advancement is thanks to a material called silicon nitride. Silicon nitride can also be produced using silicon extracted from waste solar panels, making it a promising technology that not only overcomes the limitations of fossil fuel-based processes but also offers a solution for processing discarded solar panels.
The research team led by Professor Jongbeom Baek from the Department of Energy and Chemical Engineering at UNIST announced on July 9 that they had succeeded in increasing the yield of mechanochemical ammonia production by 5.6 times using silicon nitride.
Ammonia is a crucial substance for food production, to the extent that it is said half of the world's population is sustained by ammonia fertilizers. Recently, ammonia has also attracted attention as a storage and transport medium for hydrogen, a clean fuel, and its demand is expected to grow further.
The issue lies in the method of ammonia production. For over a century, ammonia has been produced using the Haber-Bosch process. This process requires extremely high temperatures above 400°C and pressures 200 times greater than atmospheric pressure, resulting in massive energy consumption. Furthermore, the carbon dioxide emissions from this process account for more than 2% of global emissions.
This is why mechanochemical ammonia production is emerging as an alternative. In this method, steel marbles are rolled inside a sealed container, causing nitrogen (N₂) and hydrogen (H₂) molecules to collide with a catalyst and react. This approach can drastically reduce both energy consumption and greenhouse gas emissions. It is also suitable for small-scale and decentralized production, meaning ammonia can be manufactured directly at agricultural sites where it is needed.
The research team increased the ammonia yield from this process by 5.6 times compared to previous methods by adding a small amount of silicon nitride (Si₃N₄). Analysis showed that silicon nitride forms high-density defects on the surface of the iron catalyst, effectively promoting the reaction that separates nitrogen gas (N₂) into atoms and hydrogenates them.
Silicon nitride is highly resistant to impact, chemical corrosion, and heat, allowing it to maintain catalytic performance over long periods. Additionally, since it can be produced from silicon recovered from waste solar panels, it holds great potential for upcycling renewable energy waste into high-value resources. According to the International Energy Agency (IEA), more than 49 million tons of solar panel waste is expected to be generated worldwide by 2050.
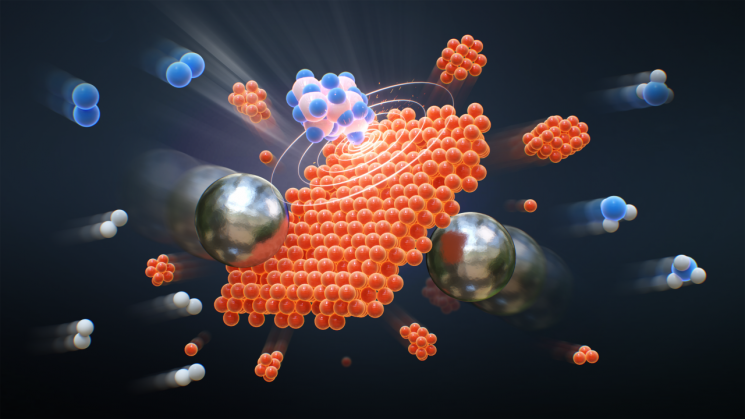 The process in which nitrogen and hydrogen react on an iron catalyst surface to produce ammonia due to the collision of steel marbles.
The process in which nitrogen and hydrogen react on an iron catalyst surface to produce ammonia due to the collision of steel marbles.
Professor Jongbeom Baek stated, "This technology can significantly improve ammonia production efficiency even at low temperatures and low pressures, contributing to the 'decentralization' of ammonia production by enabling direct production in local areas. Since it also allows for the upcycling of solar waste, it is a technology that can simultaneously address the dual challenges of decarbonizing ammonia production and resource circulation."
The results of this research were published online in the international journal Nature Communications on July 1.
This research was supported by the National Research Foundation of Korea and the Ministry of Science and ICT, among others.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















