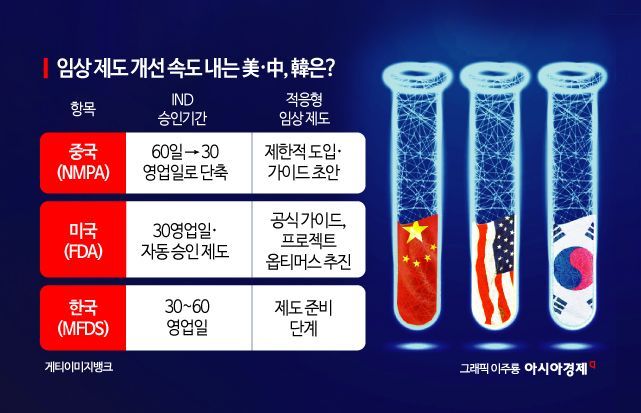
China is accelerating institutional reforms for new drug development by halving the approval period for new drug clinical trials. In contrast, while South Korea maintains international-level procedures, concerns have been raised that it may fall behind in terms of clinical approval speed and advancement. This has led to calls for the advancement of clinical trials through methods such as "adaptive trials," which allow for flexible adjustments of dosage and indications during the intermediate stages of clinical studies.
According to industry sources on July 1, the National Medical Products Administration (NMPA) of China recently announced a reform plan to shorten the review period for Investigational New Drug (IND) applications from the previous 60 business days to 30 business days. The new system will be applied first to pediatric and rare disease treatments, as well as to key strategic national new drugs. To maximize the speed of review, if there are no significant objections during the review process, approval is automatically granted. In addition, for first-class innovative new drugs, approval within 30 days and the commencement of clinical trials within 12 weeks are mandatory. China has already surpassed the United States in the number of global clinical trial registrations, resulting in global pharmaceutical companies lining up to secure clinical data in China. There are also projections that technology transfers and mergers and acquisitions (M&A) between global pharmaceutical companies and Chinese firms will accelerate even further.
The United States maintains its existing 30-business-day IND review system. The Food and Drug Administration (FDA) prioritizes safety and accuracy, aiming to reduce new drug development timelines through adaptive trial guidelines, while allowing for interim analyses and dose redesigns. In particular, the FDA is promoting "Project Optimus," an approach to determine optimal dosing for anticancer drugs, and encouraging the adoption of "seamless phase II/III" trial designs that combine phase II and phase III trials. The goal is to simultaneously shorten development timelines at each stage and minimize side effects.
Adaptive trials do not fix key variables in clinical trials but instead allow for active adjustments to dosage, dosing intervals, and patient groups based on interim analysis data. Compared to traditional fixed designs, this approach lowers the probability of failure and enables rapid transitions to clinical conditions with higher chances of success, thereby reducing both development time and costs. Global pharmaceutical companies are actively adopting this method, particularly in the development of anticancer and rare disease treatments.
An official from the pharmaceutical and biotech industry stated, "The acceleration and advancement of clinical trial approvals in China and the United States demonstrate that global competition in new drug development is becoming a key issue among countries." The official added, "South Korea must also move quickly to institutionalize and adopt adaptive trials, digital platforms, and alternative technologies to animal testing in order to achieve both speed and safety." Since a significant portion of global clinical trial costs and timelines are spent on initial approval waiting periods and step-by-step design transitions, reducing these factors can strengthen competitiveness in new drug development.
Currently, South Korea maintains an IND approval period of 30 to 60 business days. Although approvals for multinational clinical trials are processed relatively quickly, there are concerns that South Korea may fall behind China and the United States in terms of speed. The Ministry of Food and Drug Safety has operated its IND review system in accordance with international standards, but has yet to establish guidelines for innovative trial designs such as adaptive trials.
Lee Seungkyu, Vice President of the Korea Biotechnology Industry Organization, said, "The efforts of the United States and China to shorten new drug clinical trial periods are ultimately aimed at reducing costs and gaining an advantage in the new drug market." He added, "Efforts and support to reduce costs and time during the clinical review stage are also needed domestically."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.