An Average of 8,000 Monthly Prescriptions at 156 Hospitals and Clinics Nationwide
"High Proportion of Out-of-Hospital Prescriptions"... Home-Based Treatment Becoming Established
On June 23, electronic medicine platform Ybrain announced that its prescription electronic medicine for depression, 'Mindsteam,' has surpassed a cumulative total of 180,000 prescriptions.
Mindsteam is a product designed for the treatment of mild to moderate major depressive disorder and received marketing approval from the Ministry of Food and Drug Safety in 2021. In June 2022, it was selected for the Ministry of Health and Welfare's new medical technology grace period program and was designated as a non-reimbursed item, making it available as a non-reimbursed prescription medicine for depression starting in 2023.
Currently, Mindsteam is supplied to 156 hospitals nationwide, including 13 tertiary general hospitals such as Samsung Seoul Hospital, Chungbuk National University Hospital, Incheon St. Mary's Hospital, and Korea University Ansan Hospital, as well as 5 general hospitals. Severance Hospital and Korea University Guro Hospital have also introduced it for research purposes.
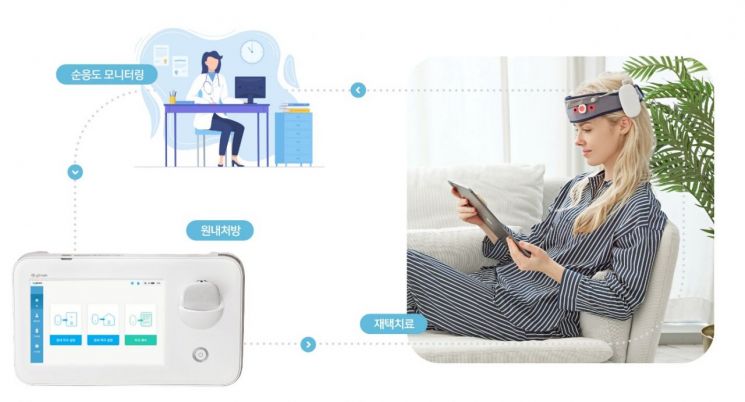
According to Ybrain, Mindsteam has an average of about 8,000 prescriptions per month, showing a steady trend in prescriptions over the past year regardless of season. Notably, the proportion of out-of-hospital prescriptions was approximately 1.5 times higher than in-hospital prescriptions, indicating that home-based treatment using Mindsteam is being stably established.
Previously, a domestic multi-center home clinical trial conducted in 2020 showed that when Mindsteam was used alone for 30 minutes daily over six weeks, the remission rate for depressive symptoms was 62.8%, which is 12.8% higher than the remission rate of conventional antidepressants (approximately 50%). In addition, interim results from an ongoing clinical study on pre- and postnatal depression at Seoul National University Hospital showed that participants treated with Mindsteam for six weeks exhibited significant improvements across all evaluation indicators.
Lee Kiwon, CEO of Ybrain, stated, "As Mindsteam has been approved as Korea's first home-use electronic medicine for depression, we hope that more active home-based treatment will help lower the barriers to depression treatment."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.