STCube, a developer of immuno-oncology drugs, announced on June 20 that it has begun dosing the first patient in a Phase 1b/2 clinical trial of its anti-BTN1A1 immune checkpoint inhibitor 'Nelmastobart' in combination therapy for patients with metastatic and recurrent colorectal cancer.
The trial targets patients who are refractory or intolerant to oxaliplatin- and irinotecan-based chemotherapy, specifically those receiving third-line or later treatments. The study focuses on microsatellite stable (MSS) colorectal cancer, where existing immunotherapy has shown limited efficacy, to evaluate the potential of BTN1A1-targeted immunotherapy.
In Phase 1b, the trial will determine the maximum tolerated dose (MTD) and the recommended Phase 2 dose (RP2D) of the combination therapy, as well as assess its safety, pharmacokinetic profile, and preliminary efficacy. The subsequent Phase 2 will select patients with high BTN1A1 expression to more precisely evaluate efficacy.
The first dosing was conducted in Phase 1b cohort 1 with two patients. The triple combination therapy employs a strategic mix of mechanisms?immune evasion inhibition, cytotoxic anticancer effects, and angiogenesis suppression?which is expected to enhance treatment potential for high-risk patients.
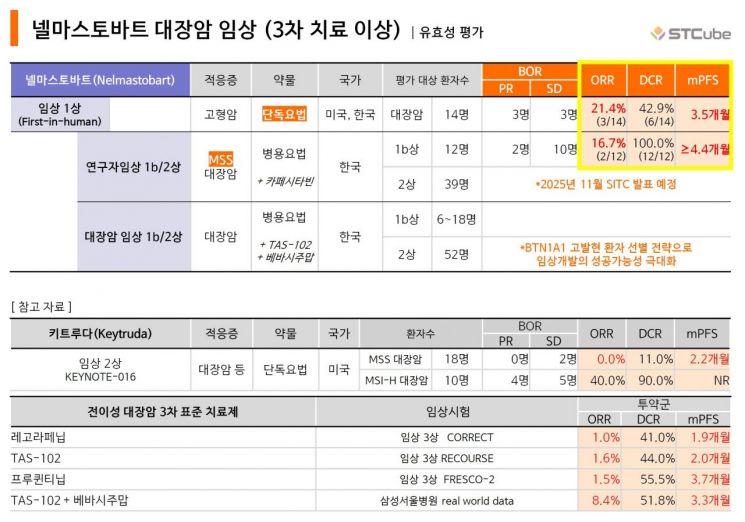
The combination of TAS-102 and bevacizumab, the drugs used in the combination therapy, was approved by the U.S. Food and Drug Administration (FDA) in 2023 as a third-line treatment for metastatic colorectal cancer. It is considered the standard of care with the best clinical outcomes for this indication. According to real-world data from Samsung Medical Center reflecting the domestic treatment environment, the TAS-102 and bevacizumab combination showed a median progression-free survival (mPFS) of 3.3 months and an objective response rate (ORR) of 8.4%, significantly improving antitumor effects compared to the TAS-102 monotherapy control group (mPFS 2.5 months, ORR 5.4%).
STCube designed this combination clinical trial as the most optimized regimen based on positive efficacy signals observed in both monotherapy and combination clinical studies. In an investigator-initiated Phase 1b trial combining Nelmastobart with the chemotherapeutic agent capecitabine, results showed an ORR of 16.7% and a disease control rate (DCR) of 100%. Encouraging therapeutic responses were also observed in MSS colorectal cancer, which is typically unresponsive to existing immunotherapies.
The triple combination strategy is expected to yield even more meaningful response rates and survival indicators in actual patient treatment. BTN1A1 expression rates are currently reported to be over 40% in colorectal cancer. The company explained that applying an appropriate patient selection strategy could not only increase clinical success rates but also provide differentiated competitiveness from a technology transfer perspective.
STCube aims to validate the clinical scalability of its BTN1A1-based precision immuno-oncology platform through this trial and plans to expand indications to various solid tumors, including lung cancer. The patient selection biomarker strategy based on BTN1A1 expression is expected to serve as a key competitive advantage in both improving treatment response rates and global technology transfer negotiations.
Jung Hyunjin, CEO of STCube, stated, "Metastatic colorectal cancer is a representative cancer type with extremely limited treatment options, and it is an area where existing immunotherapies have difficulty making an impact." He added, "The combination therapy strategy based on Nelmastobart is expected to be an effective treatment option and a new therapeutic paradigm for patients with resistant and metastatic cancer."
The Phase 1b/2 clinical trial of Nelmastobart for colorectal cancer is being conducted at five major hospitals in Korea, including Korea University Anam Hospital, Asan Medical Center, Seoul National University Bundang Hospital, Seoul National University Hospital, and Severance Hospital.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.