Development of Escherichia coli Strain for Eco-Friendly Itaconic Acid Production by UNIST, POSTECH, and KRICT
Gene Deletion Triggers Stringent Response, Unexpectedly Enhancing Metabolism; Published in Bioresource Technology
A new evolutionary technique has been developed that induces only Escherichia coli capable of more efficiently "digesting" acetic acid to survive, thereby enhancing their production capacity.
Using this approach, the resulting Escherichia coli demonstrated a 1.7-fold improvement in their ability to convert acetic acid into itaconic acid, which is used as an eco-friendly adhesive and plastic precursor.
This opens the way to stably produce chemical raw materials by utilizing Escherichia coli as cell factories.
On June 18, a research team led by Professor Donghyuk Kim from the Department of Energy and Chemical Engineering at UNIST, in collaboration with Professor Kyuyoul Jung's team from POSTECH and Dr. Myunghyun Roh from the Korea Research Institute of Chemical Technology, announced that they have developed a strain of Escherichia coli with an average 1.7-fold improvement in its ability to metabolize acetic acid into itaconic acid.
 Research team, Professor Donghyuk Kim (left) and researcher Jihoon Woo (first author). Provided by UNIST
Research team, Professor Donghyuk Kim (left) and researcher Jihoon Woo (first author). Provided by UNIST
Itaconic acid is a substance used in biodegradable plastics and medical adhesives. Currently, the standard method involves fermenting starch and other substrates with fungi, but this consumes food resources and has high production costs.
As an alternative, acetic acid?the main component of vinegar?can be used. Acetic acid is easily obtainable through various chemical processes, making it inexpensive, and if synthesized by capturing carbon dioxide, it can also contribute to carbon reduction.
However, the challenge is that microorganisms do not efficiently metabolize acetic acid. Due to its toxicity and metabolic burden, they do not grow well, and the productivity of itaconic acid drops significantly.
The research team evolved Escherichia coli by setting a condition in which only those that produce large amounts of itaconic acid survive. They inserted a biosensor into the Escherichia coli, designed so that the expression level of the antibiotic resistance gene varies according to the concentration of itaconic acid. By gradually increasing the antibiotic concentration and repeatedly culturing the bacteria, only Escherichia coli that produce high levels of itaconic acid survive.
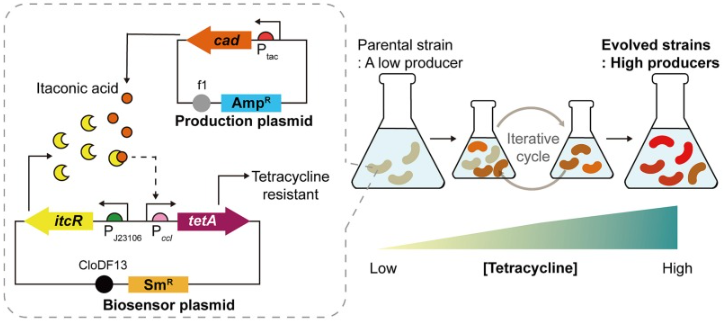 Escherichia coli with a biosensor (inside the gray dotted line) inserted. Similar to an if-then conditional statement in an electronic circuit, only the Escherichia coli that efficiently produce itaconic acid are designed to have antibiotic resistance.
Escherichia coli with a biosensor (inside the gray dotted line) inserted. Similar to an if-then conditional statement in an electronic circuit, only the Escherichia coli that efficiently produce itaconic acid are designed to have antibiotic resistance.
Through laboratory evolution over approximately 50 generations, the team obtained a strain with a 1.7-fold increase in both itaconic acid production and cell division rate compared to the original strain.
The team also performed whole-genome (DNA) and transcriptome (RNA) analyses to determine what genetic changes led to the improvements in growth and productivity. The evolved Escherichia coli had lost a genomic region corresponding to about 31,000 base pairs, and the deletion of two genes within this region was identified as the cause of improved acetic acid metabolism and growth efficiency. This gene deletion altered the physiological state of the Escherichia coli, inducing a "stringent response" typically seen under stress conditions.
Jihoon Woo, the first author of the study, explained, "The stringent response is generally described in textbooks as a mechanism that suppresses cell growth and reduces resource consumption. However, in this study, it unexpectedly improved both growth and productivity by making acetic acid metabolism more efficient."
In fact, experiments in which the relA gene was separately overexpressed also resulted in productivity improvements similar to those seen in the evolved strain. The relA gene produces ppGpp, a signaling molecule that induces the stringent response.
Professor Donghyuk Kim stated, "Through evolution-based analytical methodologies, we have reinterpreted microbial physiological responses and found clues to turning factors previously considered disadvantages into advantages. This will help in the development of sustainable chemical material production technologies that can prepare us for a future beyond fossil fuels."
This research was supported by the National Research Foundation of Korea under the Ministry of Science and ICT, and the Korea Institute of Marine Science & Technology Promotion under the Ministry of Oceans and Fisheries. The results were published in the international journal Bioresource Technology on June 1.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















