MFDS: "Carefully Check Approval and Certification Details"
The Ministry of Food and Drug Safety announced on June 17 that, after conducting a focused inspection of live commerce (online real-time commerce) broadcasts advertising food, cosmetics, and medical devices, it identified 29 cases of false or misleading advertisements that violated the Act on Labeling and Advertising of Foods, the Cosmetics Act, and the Medical Devices Act. The ministry has requested access blocks and administrative measures for these violations.
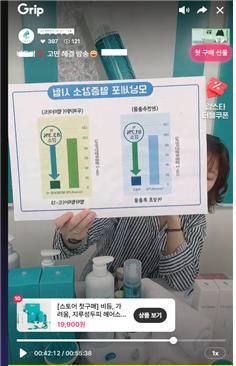 An example of a live commerce broadcast that could mislead viewers into mistaking cosmetics for pharmaceuticals. The seller used expressions such as "promotes hair growth~," "increases hair follicle cell proliferation rate," "accelerates hair follicle cell proliferation speed," and "up to 3.5 times proliferation when peptides are added compared to normally growing hair follicle cells." Ministry of Food and Drug Safety
An example of a live commerce broadcast that could mislead viewers into mistaking cosmetics for pharmaceuticals. The seller used expressions such as "promotes hair growth~," "increases hair follicle cell proliferation rate," "accelerates hair follicle cell proliferation speed," and "up to 3.5 times proliferation when peptides are added compared to normally growing hair follicle cells." Ministry of Food and Drug Safety
Live commerce broadcasts, commonly known as "Labang," is a term that combines "live streaming" and "e-commerce." These broadcasts, such as Naver Shopping Live, Kakao Shopping Live, and Coupang Live, feature celebrities and professional hosts who interact with consumers in real time to encourage purchases.
This inspection was carried out over approximately two months starting in April, in response to the rapid rise of live commerce broadcasts as a new form of e-commerce, with the aim of preventing consumer harm caused by false or misleading advertisements.
Of the violations found, there were a total of 18 cases involving food or health functional foods: 10 cases (55.6%) involved advertisements that could mislead or confuse general foods as health functional foods, such as claims about "blood sugar" or "diet"; 5 cases (27.8%) involved advertisements that could be perceived as having efficacy or effects in preventing or treating diseases, such as "constipation," "infertility," or "inflammation treatment"; 2 cases (11.1%) involved false or exaggerated claims about unapproved functionalities, such as "good for the skin"; and 1 case (5.5%) involved advertisements that deceived consumers by using testimonials.
For cosmetics, a total of 10 cases of false or misleading advertisements were discovered. These included 8 cases where advertisements could be mistaken as claiming pharmaceutical efficacy or effects, such as "helps skin regeneration" or "promotes hair growth," and 2 cases where advertisements either exceeded the scope of cosmetics, such as "filler cream," or could mislead consumers by claiming endorsement or recommendation from medical specialists, such as "product developed by a dermatologist."
In the case of medical devices, there was 1 case where a paraffin bath was falsely advertised with efficacy or effects, such as "relieves cold hands and feet," that differed from those certified.
The Ministry of Food and Drug Safety plans to continue strengthening online advertising monitoring and will closely cooperate with related organizations, such as by sharing cases of false or misleading advertisements with the Korea Online Shopping Association and requesting voluntary management.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















