BlueCare Expected to Benefit Over 1 Million Patients
First Digital Therapeutic for Depression Approved in Korea
Developed with Medical Experts; Global Expansion Planned
A path has opened for more than 1 million people suffering from depression in Korea to receive treatment via smartphone.
Hippo TNC Co., Ltd. announced on the 15th that its depression disorder treatment and management software, "BlueCare," which was developed with the participation of domestic and international medical experts, has received digital therapeutics (DTx) product approval from the Ministry of Food and Drug Safety.
This is the first time that a digital therapeutic device for the treatment of depression has received approval from the Ministry of Food and Drug Safety in Korea. In the United States, "Rejoyn," jointly developed by Otsuka and Click Therapeutics, is the only digital therapeutic device for depression that has received approval from the U.S. Food and Drug Administration (FDA).
Jung Taemyung, CEO of Hippo TNC, explained, "BlueCare, which can be used with a doctor's prescription, is a software implemented as a mobile application (app) that provides a variety of techniques for depression patients over eight weeks, including meditation, exercise, and cognitive behavioral therapy (CBT)-based conversations." He added, "Last year, 14,400 people died by suicide, giving Korea the unfortunate distinction of having the highest suicide rate among OECD countries. For those suffering from depression in our country, this will be welcome news."
CEO Jung founded Hippo TNC as a campus startup in 2020 while serving as a professor in the Department of Software at Sungkyunkwan University. The development of BlueCare, which was supported by the Ministry of Trade, Industry and Energy, involved medical experts from Samsung Medical Center, National Traffic Rehabilitation Hospital, and Asan Medical Center, among others. Professor Jeon Hongjin of the Department of Psychiatry at Samsung Medical Center, who is also the Vice Dean of the School of Medicine at Sungkyunkwan University and oversaw the pivotal clinical trial to verify efficacy and safety, stated, "Since its effectiveness has been proven in clinical trials, I expect it will be widely used in depression treatment going forward."
BlueCare is an emotional disorder treatment software based on cognitive behavioral therapy, which helps users relieve stress and discover their inner selves through conversations with a virtual dog, thereby overcoming deeply rooted feelings of depression and finding comfort. Its advantages include minimizing side effects and allowing use anytime, anywhere. After distributing the product to hospitals in Korea, the company plans to expand into global markets such as the United States, Europe, Vietnam, and Singapore.
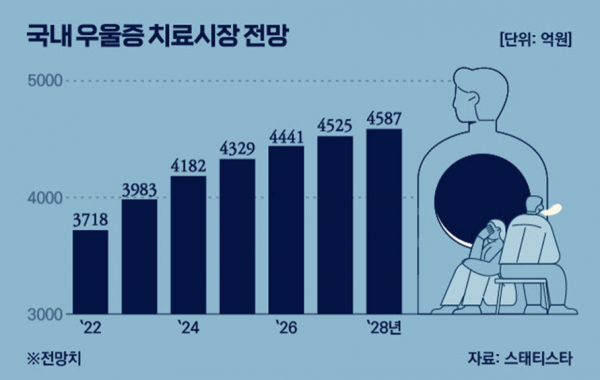
Digital therapeutics are programs that manage or treat diseases through software or apps, enabling prevention, diagnosis, and treatment outside of hospitals in daily life. As a result, the market is growing rapidly. In the United States, the market is expected to grow by an average of 30.7% per year, reaching $17.3 billion (approximately 22 trillion won) by 2030.
Previously, Hippo TNC launched the digital health product "WithBuddy" in January this year, which allows users to check for "U·Bul·S" (depression, anxiety, stress) in advance and prevent the onset of depression without visiting a hospital. This product helps strengthen mental resilience by assessing and managing an individual's psychological state starting from the stress stage.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.