Initiation of Phase 1 Combination Clinical Trial
for Patients with Advanced or Metastatic Solid Tumors
Hanmi Pharmaceutical announced on May 19 that it has entered into a "clinical trial collaboration and supply agreement" with MSD to evaluate the combination therapy of its "LAPS IL-2 analog (HM16390)" and MSD's anti-PD-1 immuno-oncology drug "Keytruda."
Hanmi Pharmaceutical will act as the sponsor and oversee Phase 1 clinical trials to assess the safety and efficacy of the combination therapy of HM16390 and Keytruda, while MSD will supply Keytruda for use in the clinical trial.
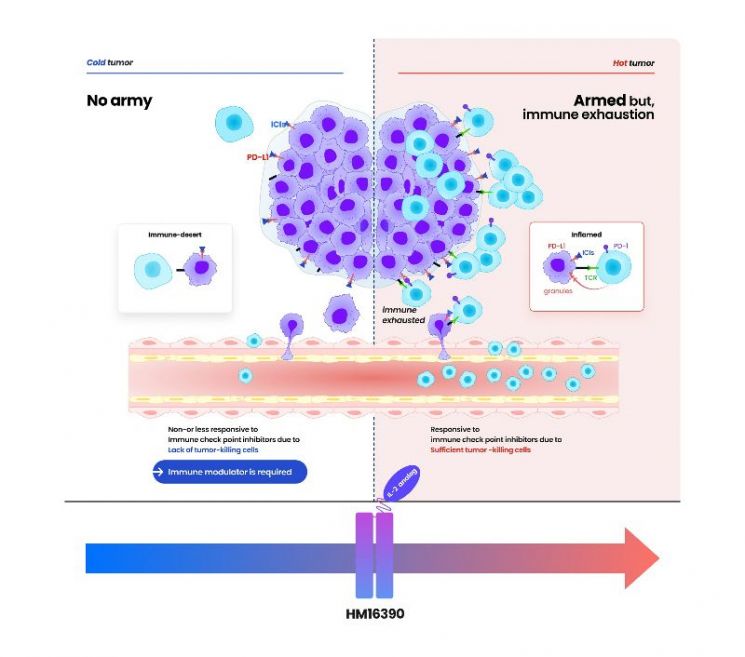
HM16390 is a next-generation IL-2 variant, newly designed with a differentiated strategy to target "IL-2 (interleukin-2)," which regulates the differentiation and proliferation of immune cells. HM16390 works by inducing the proliferation and activation of T cells. In particular, it is designed to maximize anti-tumor effects by increasing the number of tumor-infiltrating lymphocytes that respond to immune checkpoint inhibitors in the tumor microenvironment, thereby converting low-immunogenic "cold tumors" into highly immunogenic "hot tumors."
The currently approved recombinant IL-2 therapy "Proleukin" is only recommended for limited use due to side effect concerns.
If the binding affinity to the IL-2 beta receptor is reduced, side effects such as vascular leak syndrome decrease, but the anti-cancer effect is also diminished. Conversely, increasing the binding affinity to the IL-2 beta receptor and eliminating binding to the IL-2 alpha receptor enhances the anti-cancer effect, but it is known to significantly increase the risk of severe side effects such as cytokine release syndrome.
In contrast, HM16390, unlike existing IL-2 candidates, precisely modulates binding to the IL-2 alpha receptor, offering the advantage of maximizing drug efficacy while ensuring safety. Through this approach, it is expected to maintain anti-cancer effects while minimizing severe side effects.
HM16390 is being developed as a long-acting immuno-oncology drug by applying Hanmi Pharmaceutical's proprietary sustained-release platform technology "LAPSCOVERY," maximizing efficacy, safety, and duration. It is currently being developed as a long-acting therapy that can be administered subcutaneously (SC) once per cycle of anti-cancer drug treatment. Hanmi Pharmaceutical is developing HM16390 both as a monotherapy and in combination with immuno-oncology drugs for the treatment of various solid tumors, and is currently conducting a global Phase 1 clinical trial.
Roh Youngsoo, Director of Hanmi Pharmaceutical's ONCO Clinical Team, stated, "Hanmi Pharmaceutical possesses a differentiated pipeline in the oncology field, especially in immuno-oncology. Throughout this year, we will sequentially present the results of our research at various academic conferences."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.