A new type of catalyst has been developed that can produce propylene using only a tiny amount of platinum. Propylene is an essential raw material used in the production of a wide range of products, including plastics, fibers, automotive parts, and electronic devices, making it a key component of the petrochemical industry.
 Professor Min Ki Choi of the Department of Biological and Chemical Engineering (left), and Su Seong Lee, PhD candidate of the Department of Biological and Chemical Engineering (right). Courtesy of KAIST
Professor Min Ki Choi of the Department of Biological and Chemical Engineering (left), and Su Seong Lee, PhD candidate of the Department of Biological and Chemical Engineering (right). Courtesy of KAIST
On May 12, KAIST announced that a research team led by Professor Min Ki Choi of the Department of Biological and Chemical Engineering has developed a catalyst that uses only a minute amount (100 ppm, 0.01%) of platinum, based on inexpensive metals gallium (Ga) and alumina (Al2O3), and has succeeded in producing propylene using this catalyst.
Propylene is produced through a process called propane dehydrogenation (PDH), in which hydrogen is removed from propane. Until now, platinum catalysts have been widely used in this process because platinum is highly effective at breaking the bond between carbon and hydrogen and removing hydrogen. However, platinum is expensive and tends to lose its effectiveness when reused repeatedly.
To address these issues, the research team designed a catalyst based on gallium and alumina, incorporating only a very small amount of platinum. Notably, this new catalyst outperforms commercial catalysts that use high concentrations of platinum (10,000 ppm).
The core principle of this new catalyst is that gallium activates the carbon-hydrogen bond in propane to remove hydrogen and generate propylene, while platinum combines with the remaining hydrogen atoms on the surface, converting them into hydrogen gas (H2) and removing them from the catalyst surface.
By utilizing this principle, the roles of the two metals are divided, allowing for a significant reduction in platinum usage while maintaining high performance.
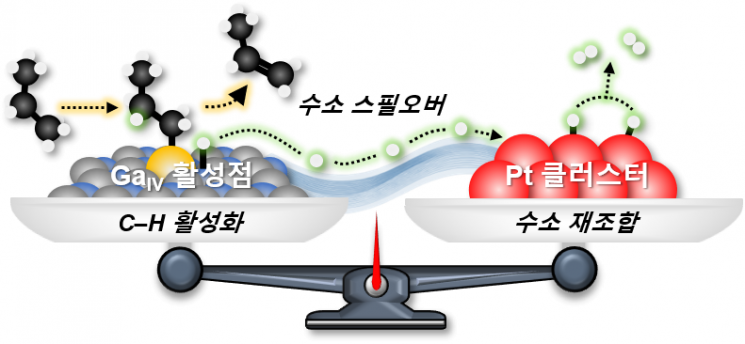 Schematic diagram illustrating the catalytic collaboration between gallium (Ga) and platinum (Pt). Provided by KAIST
Schematic diagram illustrating the catalytic collaboration between gallium (Ga) and platinum (Pt). Provided by KAIST
The research team also demonstrated that the optimal performance is achieved when the ratio of platinum to gallium is appropriate. In addition, they provided a scientific explanation for the ideal compositional ratio and presented a quantitative indicator that allows for prediction.
The team solved the issue of "sintering," in which platinum particles clump together and performance drops sharply when platinum is reused multiple times. By adding a small amount of cerium (Ce), the researchers successfully suppressed the aggregation of platinum particles and confirmed that the catalyst maintained stable performance even after more than 20 cycles of reaction and regeneration.
Professor Min Ki Choi stated, "This study is significant in that it demonstrates the possibility of reducing platinum usage to just 1/100 of conventional levels while maintaining or even improving performance. Based on this, we can expect simultaneous economic and environmental benefits, such as reduced catalyst costs, longer replacement cycles, and decreased waste catalysts."
He added, "The research team plans to review the feasibility of large-scale process demonstrations and commercialization in the future. If the new catalyst developed by our team is actually applied in industrial settings, it will further improve the economic viability and efficiency of propylene production."
This research was supported by the National Research Foundation of Korea and Hanwha Solutions Co., Ltd. The results of the study (with Professor Min Ki Choi as corresponding author and Su Seong Lee, PhD candidate, as first author) were recently published in the Journal of the American Chemical Society, a leading journal in the fields of chemistry and chemical engineering.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.











